It is possible to convert CO 2 into fuels like methane using light. A Chinese research team has created a photocatalyst based on gold atoms that can be used to transform things.
The photocatalytic conversion of CO 2 takes place through several processes. Carbon Monoxide, Methanol, methane, and other hydrocarbons can be produced by this. The transfer of eight electrons from CO 2 to CH 4 is more than for other products. The competing reaction to form CO only requires two electrons and is much quicker than methane, so it is favored. It is particularly difficult to effective andselective methanization.
An approach to convert CO 2 to CH 4 using solar energy has been developed by a team of people. Their success is due to a novel catalyst with gold atoms. A new strategy that uses a complex exchange to produce the catalyst was developed because gold atoms aggregate.
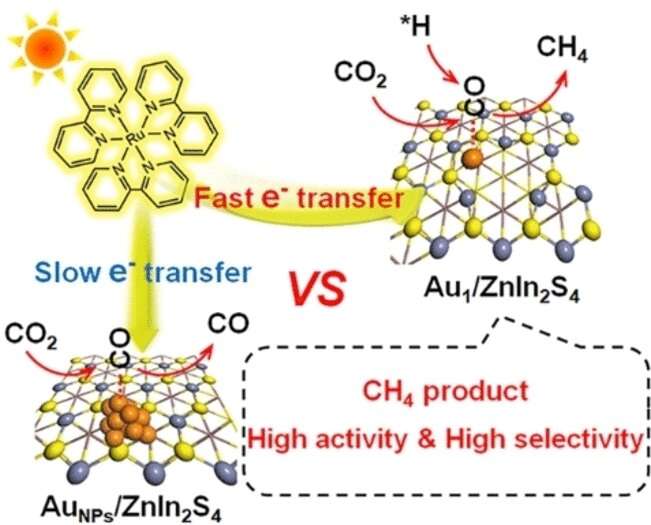
The electronic structures of single-atom catalysts make them behave differently than metal nanoparticles. Almost all single atoms can be used as active catalytic centers. In this new catalyst, single gold atoms are anchored to an ultrathin zinc–indium sulfidenanolayer and are coordinated to only two sulfur atoms. The catalyst was shown to be very active under the sun.
An electron that is made available by an electron donor is accepted by a photosensitizer. The electron goes to the catalyst. There are gold atoms on the support. The electrons are captured more effectively by them than by gold nanoparticles.
The catalyst activated the CO 2 molecule to a much greater degree than gold nanoparticles, more strongly adsorbed the excited CO intermediates, and lowered the energy barrier for binding hydrogen ion. CH 4 is the preferred product and the release of CO is minimized.
More information: Shenghe Si et al, Low‐Coordination Single Au Atoms on Ultrathin ZnIn 2 S 4 Nanosheets for Selective Photocatalytic CO 2 Reduction towards CH 4, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202209446