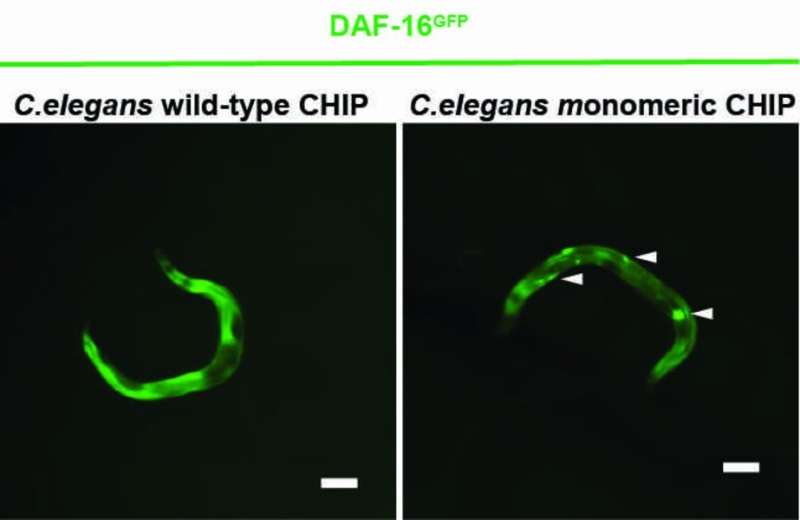
A new study shows that the CHIP is more efficient in regulating the insulinreceptor alone than it is in a pair. In cellular stress situations, CHIP usually serves to degrade misfolded and deficient proteins. The cell is cleaned up by CHIP. CHIP works with helpers to attach a chain of ubiquitin to misfolded proteins The cell is able to eliminate the defects by recognizing them. The CHIP regulates the signal transduction of theinsulinreceptor The ubiquitin is degraded by CHIP and the life-extending genes are stopped.
Experiments with Caenorhabditis elegans and human cells show that CHIP can be labeled with ubiquitin, which prevents it from forming a dimer. The CHIP is more efficient than the CHIP dimer. The University of Cologne's cluster of excellence for cellular stress responses in aging-associated diseases published a study called "A Dimer-Monomer switch controls CHIP-Dependent Substrate Ubiquitylation and Processing"
It depends on the state of the cell. Vishnu Balaji is the first author of the study.
CHIP can also mark the helpers for degradation after cleaning up the defects. He said that this allows CHIP to function as a monomer again. There needs to be a balance between the dimeric and monomeric states of CHIP.
The balance of CHIP seems to be disrupted in diseases of the brain. There are different sites of CHIP that function differently in spinocerebellar ataxias. There is a possibility of shifting to more monomers being a therapeutic approach.
In the next step, the scientists want to find out if there are otherproteins orreceptors that the CHIP monomer binding to regulate their function. In order to be able to develop more targeted therapies in the future, the researchers are interested in finding out which tissues and organs have more and less CHIPs.
More information: Vishnu Balaji et al, A dimer-monomer switch controls CHIP-dependent substrate ubiquitylation and processing, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.08.003 Journal information: Molecular Cell