A team of researchers led by Meenesh Singh at the University of Illinois Chicago have found a way to convert carbon dioxide captured from industrial exhaust into ethylene, a key building block for plastic products.
Their findings are published in a journal.
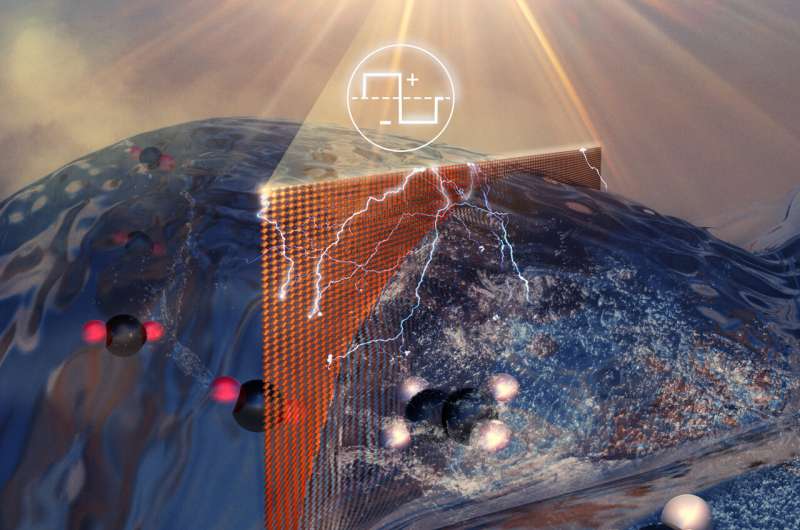
The UIC team's approach is the first to achieve nearly 100% utilization of carbon dioxide to produce hydrocarbons, a feat that has been explored for more than a decade. Their system uses electrolysis to convert captured carbon dioxide gas into high purity ethylene.
The process can recycle almost all carbon dioxide captured. The process can be carbon negative if renewable energy is used.
Singh's approach surpasses the net-zero carbon goal of other carbon capture and conversion technologies by actually reducing the total carbon dioxide output from industry. He stated that it was a net negative. 6 tons of CO 2 are taken from point sources that otherwise would be released to the atmosphere for every 1 ton of ethylene produced.
The source carbon dioxide emission stream has been used to convert carbon dioxide into ethylene. Less than 10% of CO 2 emissions usually convert to ethylene. In an energy intensive process, the carbon dioxide and the ethylene must be separated.
An electric current is passed through a cell, half of which is filled with carbon dioxide, the other half with a water-based solution. A catalyst draws charged hydrogen atoms from the water molecule into the other half of the unit, where they are combined with charged carbon atoms from the carbon dioxide molecule to form a compound called ethylene.
After ammonia and cement, ethylene is the third most carbon emitting chemical. In addition to creating plastic products for the packaging, agricultural and automotive industries, ethylene is also used to produce chemicals used in antifreeze, medical sterilants and vinyl siding for houses.
It takes a lot of heat to make ene in steam cracking. About 1.5 metric tons of carbon emissions are generated by cracking. More than 260 million tons of carbon dioxide emissions are caused by the production of 160 million tons of ethylene.
The scientists at the University of Illinois at Chicago were able to produce other carbon-rich products using their electrolysis approach. They were able to convert 10% of the solar energy into carbon product output. State-of-the-art standard is 2%. The solar energy conversion efficiency was 4% for all the ethylene they produced.
More information: Aditya Prajapati et al, CO2-free high-purity ethylene from electroreduction of CO2 with 4% solar-to-ethylene and 10% solar-to-carbon efficiencies, Cell Reports Physical Science (2022). DOI: 10.1016/j.xcrp.2022.101053 Journal information: Cell Reports Physical Science