electron orbitals show where and how electrons move They have been used as a model for quantum mechanical description in chemistry and physics. When two substances react with each other, this is what happens. Researchers at Forschungszentrum Jlich and the University of Graz have found that the course of chemical reactions is dependent on the distribution of momentum space. Results were published in a journal.
The formation and breakdown of electron bonds is what chemical reactions are all about. Predicting the path of chemical reactions can be done with the help of the so-calledMolecular Orbital Theory. The widespread use and application of the method was caused by the fact that it was greatly simplified by Kenichi Fukui and Roald Hoffmann.
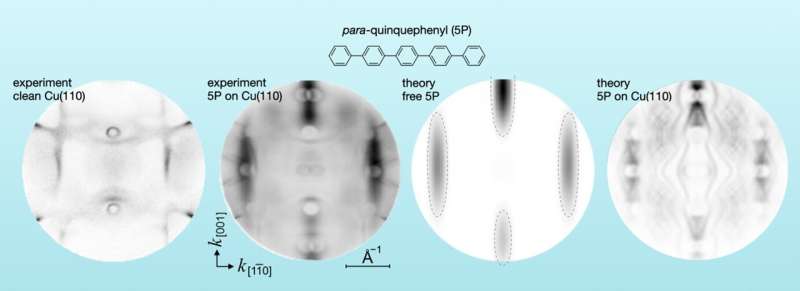
The energy and location of electrons are studied. The photoemission method was used to look at the orbitals. He collaborated with his colleagues at the Peter Grnberg Institute in Jlich and the University of Graz to map the measured momentum in the so-called momentum space.
Predicting emission from many different molecule on metals is possible. One can use a free molecule that doesn't interact with metal as a model. We realized that the experimental result was different from the theoretical predictions when we measured the oligophenyls. Some parts of the space were not occupied. The band gaps of electronic states are usually found in noble metals. One of the noble metals is copper.
The work was done at the Elettra synchrotron in Italy. An international consortium led by Forschungszentrum Jlich operates a photoemission electron microscope at a beam line. The work was done with Professor Michael. Professor Peter Puschnig is from the University of Graz. Peter Puschnig gave the key to explaining the selection criterion with his quantum mechanical simulations.
More information: Xiaosheng Yang et al, Momentum-selective orbital hybridisation, Nature Communications (2022). DOI: 10.1038/s41467-022-32643-z Journal information: Nature Communications