Hydrogen is considered to be an ideal energy carrier. The highest gravimetric energy density of all chemical fuels is found in hydrogen. Current storage options require a lot of space, so it's not widely used in transportation applications.
H2O is a gas and can be found in a volume of 12,000 liters. The volume of hydrogen in a fuel-cell vehicle is reduced to 25 liters per kilogram because of the high pressure. Since the boiling point of hydrogen is minus 253 C, liquid hydrogen requires very low temperatures.
A group of scientists have shown that hydrogen can condense on a surface at a very low temperature.
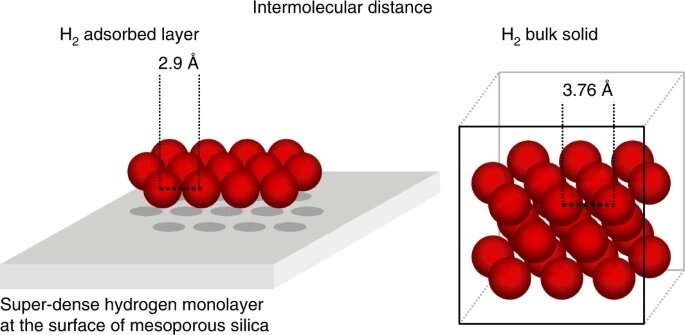
Even though both the noble gas argon and the H 2 molecule are the same size, the H 2 molecule covered the surface. H 2 molecule squeezes closely together to form a super- dense layer.
A study by R. Balderas-Xicohténcatl et al. involved high-resolution cryo-adsorption experiments on highly-ordered mesoporous silica.
This two-dimensional hydrogen layer can be followed with inelastic neutron scattering. This hydrogen was confirmed for the first time. The inelastic count rate on the VISION is more than 100 times greater than any other available instrument.
Experiments show the high hydrogen density in the adsorbed layer. The attractive forces at the surface were more powerful than the repulsion between the two hydrogen molecule. The high compressibility of hydrogen causes the high density.
The formation of the hydrogen layer at low temperatures is of interest. It could be used for quantitative analysis of H 2 at 20 K.
NatureChemistry published the research.
More information: Rafael Balderas-Xicohténcatl et al, Formation of a super-dense hydrogen monolayer on mesoporous silica, Nature Chemistry (2022). DOI: 10.1038/s41557-022-01019-7 Journal information: Nature Chemistry