Many natural and engineered materials have an entire universe hidden within them. The chemistry of the materials can be different when they are submerged in liquid.
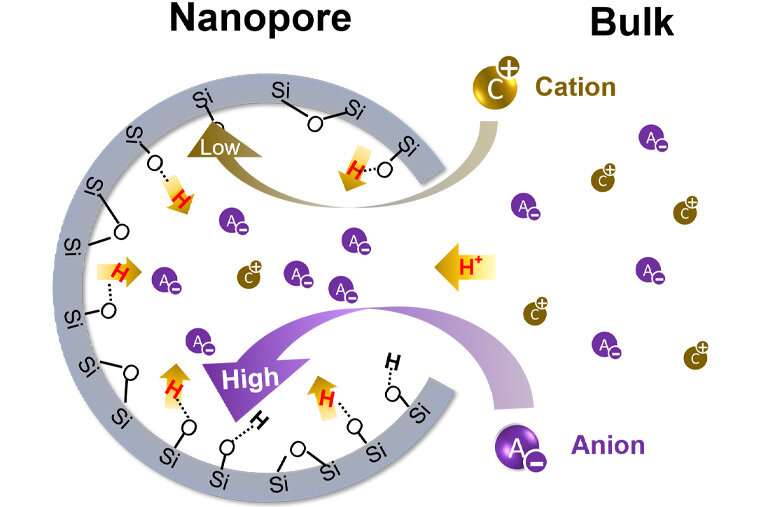
The pH inside of nanopores can be as much as 100 times more acidic than in the bulk solution.
The findings were published in a journal.
There are a variety of engineering processes that can be affected by a better understanding of the particles in the air. Decarbonization technologies for energy systems include carbon capture and sequestration, hydrogen production and storage, and batteries.
Young-Shin Jun, a professor of energy, environmental and chemical engineering, and Srikanth Singamaneni, a professor of mechanical engineering and materials science, wanted to understand how pH is.
Jun said thatpH is a master variable for water chemistry. The pH of the bulk solution is not the same as the pH inside the material's nanopores.
If they are different, that will affect the entire prediction in the system.
Jun and her former classmates worked with Singamaneni and his students. The plasmonic sensor developed by Singamaneni showed how the pH changed as it moved through a biological system. The kind of sensor Jun could use is a gold nanoparticles and a molecule that is sensitive to the pH.
The pH of the immediate environment is reported when light shines on the probe molecule. It's hard to detect the weak signal offered by normal raman scattering. The gold nanoparticles act as an antenna and amplify the effect.
In order to measure the pH innanopores, Singamaneni encased a sensor in asilica shell with just three nanometers in diameter and put it into liquid solutions with different chemistries The team was able to verify that the sensors only provided chemical information from inside theSiO2 and were not contaminated by the bulk solution.
Because the gold nanoparticles amplify the scattering of molecule only in their immediate vicinity, they can also give information about the ion and molecule inside the pores.
The probe molecule is not detecting the change in the pH outside thenanopore. It's only watching what's happening in the environment.
In the lab, the research teams found that the negatively charged ion was preferentially transported into the nanopores.
The higher the solution's salt content, the greater the difference. This could apply to brines from desalination plants, oil and gas recovery, or geologic carbon sequestration. Higher reactivity in processes can be achieved by engineered materials.
This finding may help explain why engineering processes don't always agree with predicted outcomes.
Jun said that the power of this gave them. We'd been using information from the bulk systems. We thought the chemistries involved in the bulk solution and the solution in nanopores were the same, but we were wrong.
More information: Yaguang Zhu et al, Ionic surface propensity controls pH in nanopores, Chem (2022). DOI: 10.1016/j.chempr.2022.07.021 Journal information: Chem