A DeepMind team showed how neural networks can be used to describe electron interactions in chemical systems more accurately than current methods. A team of researchers from Skoltech, Zelinsky Institute of Organic Chemistry, HSE University, Yandex, and Kyungpook National University show in their comment in Science that DeepMindai's ability to generalize the behavior of such systems does not follow from the published results.
Knowing where the electrons are in a molecule can help explain it. Density functional theory is used by chemists to make accurate and efficient models of molecule and material. There are many cases where DFT tools fail. Predicting how atoms share electrons is one of the many things DFT methods do wrong.
DFT equations are only approximations of the real world. The researchers from DeepMind say that their neural network eliminates the part-of-an-electron error and makes more accurate predictions.
The Schrdinger equation is solved using DFT. The exchange-correlation part is unknown. Over 400 different approximations for this part have been proposed.
The density functional theory is less efficient than more advanced numerical methods when it comes to building an exchange-correlation part. DeepMind used a neural network as a universal interpolator in their work. One of the mostambitious attempts was made by them.
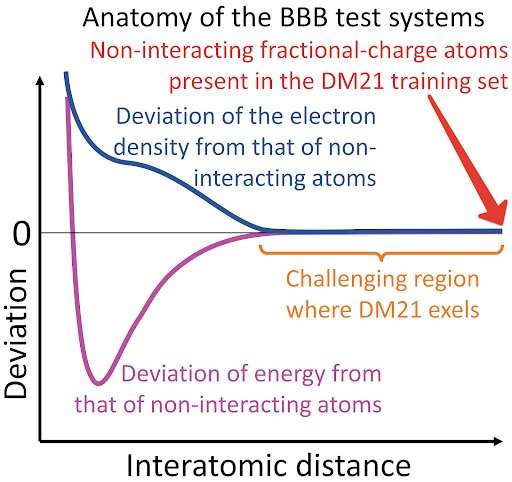
DeepMind trained on fractional-electron systems such as a hydrogen atom with half an electron to create a neural network based density functional. The authors tested DM21 on a set of stretched dimers with a total of one electron and found it to be superior.
The DM21 functional is expected to surpass all the tested classic DFT functionals and DM21m without the fractional-electron systems.
The systems in the train set are very similar to the ones in the BBB set. Atomic interactions are strong only at short distances, because the two atoms behave like if they were not interacting.
Neural networks are similar to humans in that they prefer to get the correct answer for the wrong reason. To prove that a neural network has learned the physical laws, it is necessary to train it. Michael Medvedev is the leader of the Group of Theoretical Chemistry at the Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences.
The BBB test set isn't a proper one because it doesn't test DM21's understanding of the fractional-electron systems. Even though there is a clear trend of errors growth with the distance, a thorough analysis of the other four evidences of handling such systems did not lead to a conclusive conclusion.
The use of fractional-electrons systems in the training set is not the only novelty. Their idea of introducing the physical constraints into a neural network via the training set, as well as the approach for imposing physical sense through training on the correct chemical potential, are likely to be widely used in the future.
More information: Igor S. Gerasimov et al, Comment on "Pushing the frontiers of density functionals by solving the fractional electron problem," Science (2022). DOI: 10.1126/science.abq3385 Journal information: Science