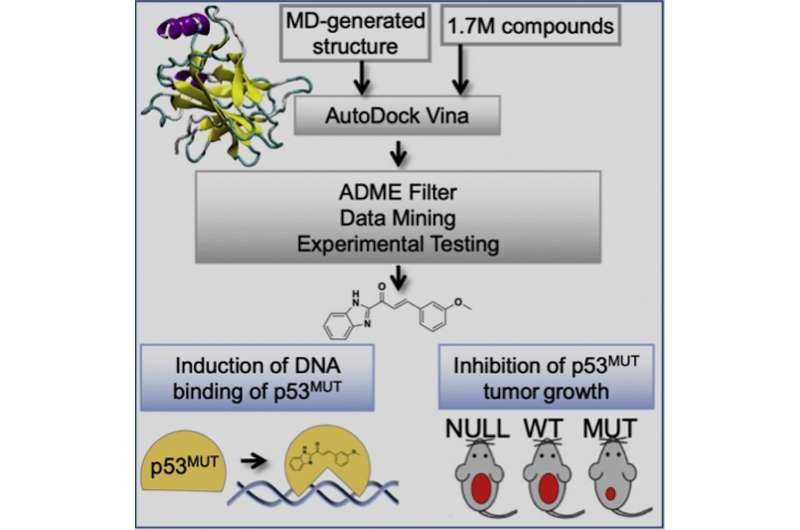
A new study, led by researchers from the University of California, Irvine and the University of California, San Diego, reveals a new computation-guided approach to identify smallmolecules that can restore parts of wild-type p53 tumor suppression function. The approach was successful in both petri dishes and in the body. The strategy can increase the chemical diversity of correctors.
One of the most powerful mechanisms organisms use to protect themselves from cancer is the Tumor Suppressor p 53. Elephants don't get cancer because they have many copies of the p 53 gene. Humans have a single copy of the most common cancer-causing genes. Therapeutic approaches are being pursued to target this pathway.
Peter Kaiser, professor and chair of the Department of Biological Chemistry, said that a lot of p53 alterations are missense, where the genetic code of the p53 is altered in a way that produces a different amino acid than it would normally. This results in a lot of p53 levels in tumors that can be treated with a corrector drug.
The study found small drug-like compounds that act through a well-defined mode of action, do not require covalent attachment, and have anti-cancer activities on tumors with p53 missense genes. The framework for p53 reactivation compound discovery provided by this research can help to increase chemical diversity and improve pharmacological properties needed for translation of pharmaceutical p53 reactivation to the clinic.
Kaiser said that the study demonstrated the feasibility and efficacy of pharmaceutical reactivation. Given the large number of cancer patients that could benefit from such drugs, these findings are positive.
A virtual screening approach developed in the lab of the endowed chair in the Department of Chemistry and Biochemistry at UC San Diego has the potential to identify compounds with increased cancer killing potential. The researchers showed that their compounds bind p53 and change it to different types of structures. Tumor progression in mouse models can be prevented with the help of this restoration of p53 binding activity.
Defining exact mechanisms and developing highly active corrector drugs for p 53 are some of the challenges that need to be solved.
More information: Geetha Durairaj et al, Discovery of compounds that reactivate p53 mutants in vitro and in vivo, Cell Chemical Biology (2022). DOI: 10.1016/j.chembiol.2022.07.003 Journal information: Cell Chemical Biology