
A new pathway for how sulfur particles can form in the atmosphere of Venus has been identified by scientists. The identity of the ultraviolet absorber on Venus is a mystery.
The atmosphere of Venus contains SO 2 and sulfuric acid. Sulfur particles are expected to be produced by the destruction of SO 2. They are built up from atomic S to S 8. James Lyons, an author on the Nature Communications paper "chemical and thermochemical pathways to S 2 and polysulfur formation in the atmosphere of Venus", asked how this process was initiated.
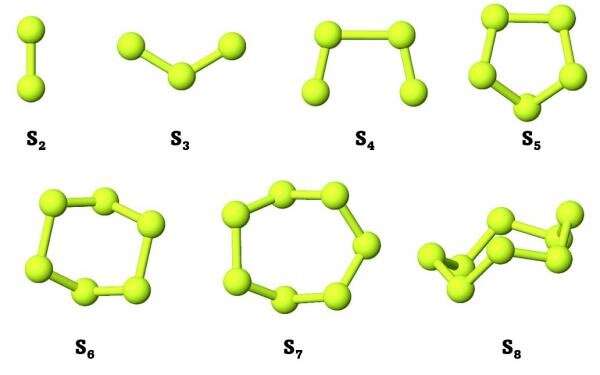
S 2 and S 2 can be formed from two sulfur atoms. Sulfur particles can be formed either by condensation of S 8 or by condensation of S 2, S 4 and other allotropes.
The sulfur particles are made up of mostly S 8 and have a ring structure. S 8 is more stable than the other allotropes because of the ring structure. We can either start with two S atoms and make S 2 or we can start with another pathway and make S 2.
The new pathway for S 2 formation is the reaction of sulfur monoxide and disulfur monoxide, which is much faster than combining two S atoms to make S 2.
Computational chemistry techniques are being used for the first time to determine which reactions are most important, instead of waiting for laboratory measurements to be done. Lyons said that this approach is needed to study the atmosphere of Venus. People are reluctant to go in the lab to measure rate constants for compounds that are hard to work with. Computational methods are the best alternative.
Computational methods were used to compute the rates. These are state-of- the-art models. The calculations were made by the authors from Spain and the University of Pennsylvania.
The research shows another pathway to sulfur particle formation. The formation of the UV absorber is likely to be aided by sulfur chemistry in Venus' atmosphere. The work opens the door to using techniques to disentangle the chemistry of Venus.
More information: Antonio Francés-Monerris et al, Photochemical and thermochemical pathways to S2 and polysulfur formation in the atmosphere of Venus, Nature Communications (2022). DOI: 10.1038/s41467-022-32170-x Journal information: Nature Communications