The method for the precise, flexible modification of a broad class of chemical compounds called bicyclic aza-arenes has been developed by chemists from the University of California, Los Angeles.
The landmark achievement, reported August 9, 2022, in Nature, shows a powerful new approach that makes it easier and more flexible for chemists to make many chemical products, including potential blockbuster drugs.
"These new methods effectively give chemists a unified, practical, late-stage'molecular editing' toolkit for modifying bicyclic aza-arenes at desired sites in any desired order--greatly expanding the diversity of drugs and other useful molecule that could be built from these popular starting compounds
Yu and his lab collaborated with the lab of a professor at UCLA. The Yu and Houk labs had the first two authors of the study.
It has always been more difficult to build things at macro scale than it has been to build things in a lab. A mix of forces governs how atoms move and bond to each other. Although chemists have developed hundreds of reactions that can transform starting compounds into other compounds, they don't have the toolkits to modify widespread carbon centers.
Synthetic chemists want to develop methods that can modify as many carbon atoms as possible at any site by breaking carbon-hydrogen bonds in the starting molecule. Synthetic chemists wanted to modify the carbon atoms on the back of an organic molecule in a way that made it easier to modify more than one carbon atom on the molecule.
The construction of new molecule would be as easy as changing individual words at will. It's difficult to make reactions that can direct a modification to one specific atom and not others that may be virtually identical in traditional chemical terms.
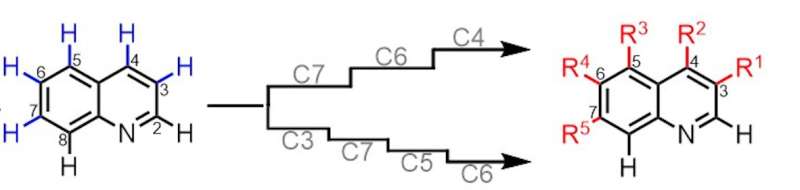
This dream has been turned into a reality thanks to the new method. Bicyclic aza-arenes are mostly made of carbon atoms and have one nitrogen atom. Bicyclic aza-arene scaffolds are used to build drugs and compounds.
The new methods allow the modification of multiple carbon atoms when they are bound to simple hydrogen atoms. Novel, potentially pharmaceutically relevant structures were previously difficult to synthesise.
The new methods remove a standard hydrogen atom from a carbon atom and replace it with a more complicated set of atoms. The Yu laboratory is known for its many innovations in the field of CH functionalization.
Like construction cranes, the new methods use directing templates that anchor to the starting molecule and direct CH functionalization at the desired sites. The templates are consideredcatalytic because they direct the reactions but are not consumed by them and thus continue to work.
Yu says that the templates direct CH functionalization not based on traditional electronic criteria but on the distance and geometry of the path to the target.
The pharma industry and other chemistry-based industries should be the first to adopt the new set of techniques.
Yu expects to broaden this approach to other classes of starting compounds.
More information: Fan, Z. et al. Molecular Editing of Aza-arene C–H Bonds by Distance, Geometry and Chirality, Nature (2022). DOI: 10.1038/s41586-022-05175-1 Journal information: Nature