The National Institute of Health has developed a three-dimensional structure that allows them to see how and where disease can lead to mitochondrial diseases. Our bodies use energy from food to convert it into theprotein. Prior to the development of this 3D structure, researchers only had models and were not able to figure out how these genes contribute to disease. One in 5000 people are affected by a group of inherited conditions that have very little treatment.
The National Institute of Environmental Health Sciences' Mitochondrial DNA Replication Group is part of the study. "Clinicians can now use this information to help find causes and help families make decisions about having more children, as well as helping them decide if they want to have more children."
The new findings will be relevant for the development of targeted treatments for patients who suffer from diseases such as progressive external ophthalmoplegia, a condition that can lead to loss of muscle functions involved in eye and eyelid movement, as well as Perrault syndrome, a rare genetic disorder that can cause
The twinkle structure and all the coordinates can be found in the open data data bank.
Researchers have been trying to understand the structure of twinkle for a long time. William C. Copeland is the corresponding author on the paper. Using the best equipment in the world, we were able to build the last missing piece for the human mitochondria.
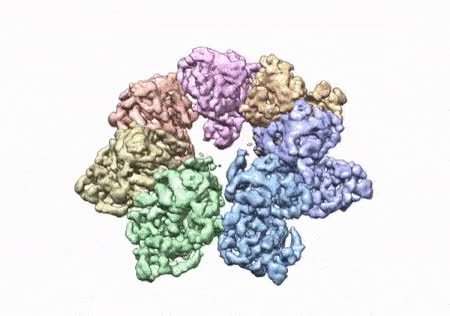
The researchers were able to see the intricate structures of hundreds of amino acids and how they interact with each other with the use ofCryoEM.
Mitochondria, which produce energy, are at risk of being disrupted. Mitochondria have their own genetic material. There are two copies of each of the chromosomes in the nucleus of a cell. A high number of chromosomes allows the cell to tolerate a few changes, but too many can lead to diseases.
To solve the structure, the researchers used a clinical mutations known to cause ophthalmoplegia. They were able to see thousands of particles in different orientations. A multi-protein circular arrangement is shown in the last structure. They used mass spectrometry and computer simulations to understand the cause of disease.
They were able to find up to 25 diseases. They found that many of the diseases map right at the junction of the two subunits, suggesting that they would be weakened by the disease.
The arrangement of twinkle is not easy to understand. The twinkle pieces may no longer fit together properly due to a clinical mutation.
Matthew J. Longley said that the structure of Dr. Riccio and the team's work allowed them to see so many of the disease genes assembled in one place. It's very rare to see a paper that explains so much. We are one step closer to having the information we need to develop treatments for these diseases thanks to this work.
More information: Amanda A. Riccio et al, Structural insight and characterization of human Twinkle helicase in mitochondrial disease, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2207459119 Journal information: Proceedings of the National Academy of Sciences