The gold standard for detecting the presence of the coronaviruses that give rise to COVID-19 is the use of the polymerase chain reaction test. They can take a few days to process, which can cause delays for people who need proof of negative testing. It's convenient, but less reliable than the other tests.
Penn State researchers have created an at- home saliva-based testing platform that can provide results in 45 minutes. The platform was able to detect the COVID-causing viruses with the same level of sensitivity. The results were published this week.
"PCR test results take about an hour to develop in a lab, but you have to factor in the time it takes to send the sample to a lab and for the lab to process it," said the principal investigator. We wanted to make a viable alternative for people to use at home without having to go to the doctor.
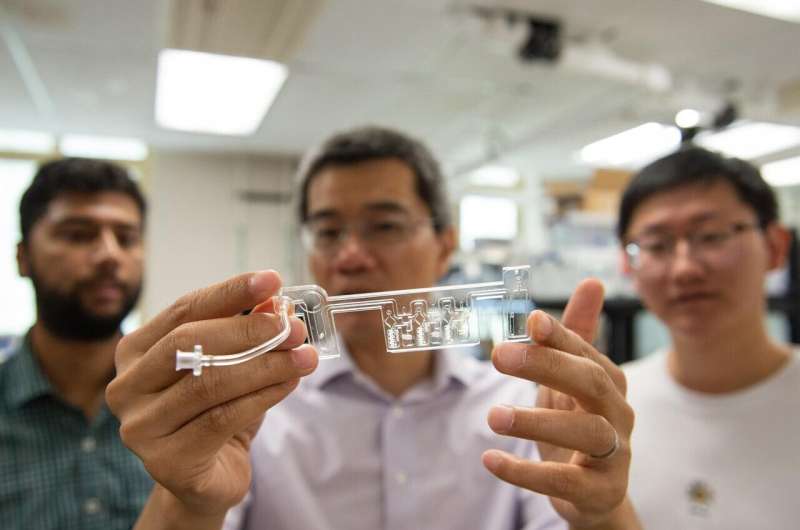
The palm-sized testing kit was developed by the team. The test results are sent within 45 minutes.
The platform uses isothermal amplification to detect viruses. The saliva is heated to 203 degrees F, the temperature at which viral particle shells break apart. Pre-packaged reagents are mixed with the genetic material in a device. After the sample is cooled to 149 degrees, a chemical reaction in which a few viral molecules are multiplied into billions of copies makes it easier to identify the virus. The user will get a positive result if the virus is present in the saliva sample
To test the setup, Guan and his team infused human saliva samples purchased commercially with inactivated SARS- CoV-2 virus particles and ran the samples through a prototype. Some of the clinical specimen were tested. The platform was able to determine if a sample was positive or negative.
The quantity of COVID particles in each mock sample was controlled. Our platform was sensitive to the presence of the virus in both the mock and clinical samples, with the standards set by thePCR test as our benchmark.

The researchers plan to continue testing their platform with more clinical COVID samples through a collaboration with the medical director of the clinical chemistry and automated testing laboratory at Penn State.
The researchers are trying to improve the test's shelf life as the prototype degrades at room temperature within three days of production. reagent lyophilization can be used to extend the shelf life of biological material. The method will allow the test to last at least six months at room temperature, according to preliminary results.
The researchers applied for a patent on their device and said they plan to market it.
The clinical samples were acquired by the interim director of Penn State's animal diagnostic laboratory. Some of the contributors include a Penn State graduate student in electrical engineering and a Penn State undergraduate student in electrical engineering.
More information: Zifan Tang et al, SLIDE: Saliva-Based SARS-CoV-2 Self-Testing with RT-LAMP in a Mobile Device, ACS Sensors (2022). DOI: 10.1021/acssensors.2c01023 Journal information: ACS Sensors