The best way to see an atom is as a dense nucleus surrounded by electrons. The picture immediately raises a question: How do electrons stay around the nucleus?
The development of quantum mechanics was the result of a search for the answer to this burning question.
Physicists began to put together a picture of the atom after many experiments. Each atom has a dense, heavy, positively charged nucleus surrounded by a cloud of tiny, negatively charged electrons. Their next step was to make a more detailed model.
Weird 'gravitational molecule' could cause black holes to spin around.
RECOMMENDED VIDEOS FOR YOU...
The solar system has a dense nucleus with a cloud of smaller particles around it. Two major problems were introduced by this model.
There is a particle that emits radiation. electrons are charged particles and they accelerate This emission would cause the electrons to lose energy and spiral in and out of the nucleus. Physicists thought that an inward spiral would take less than a second. This wasn't going to work since atoms live a long time.
There was a second issue with the nature of the radiation. Scientists have known for a long time that atoms emit radiation. If an electron followed the solar system model, it would emit all sorts of wavelength differently.
The first person to come up with a solution to this issue was a physicist from the Danes. In 1913, he suggested that electrons in an atom couldn't just have a certain amount of space in their nucleus. They had to be locked into a specific position at a certain distance from the nucleus. He proposed that an electron could not move closer to the nucleus than a certain distance.
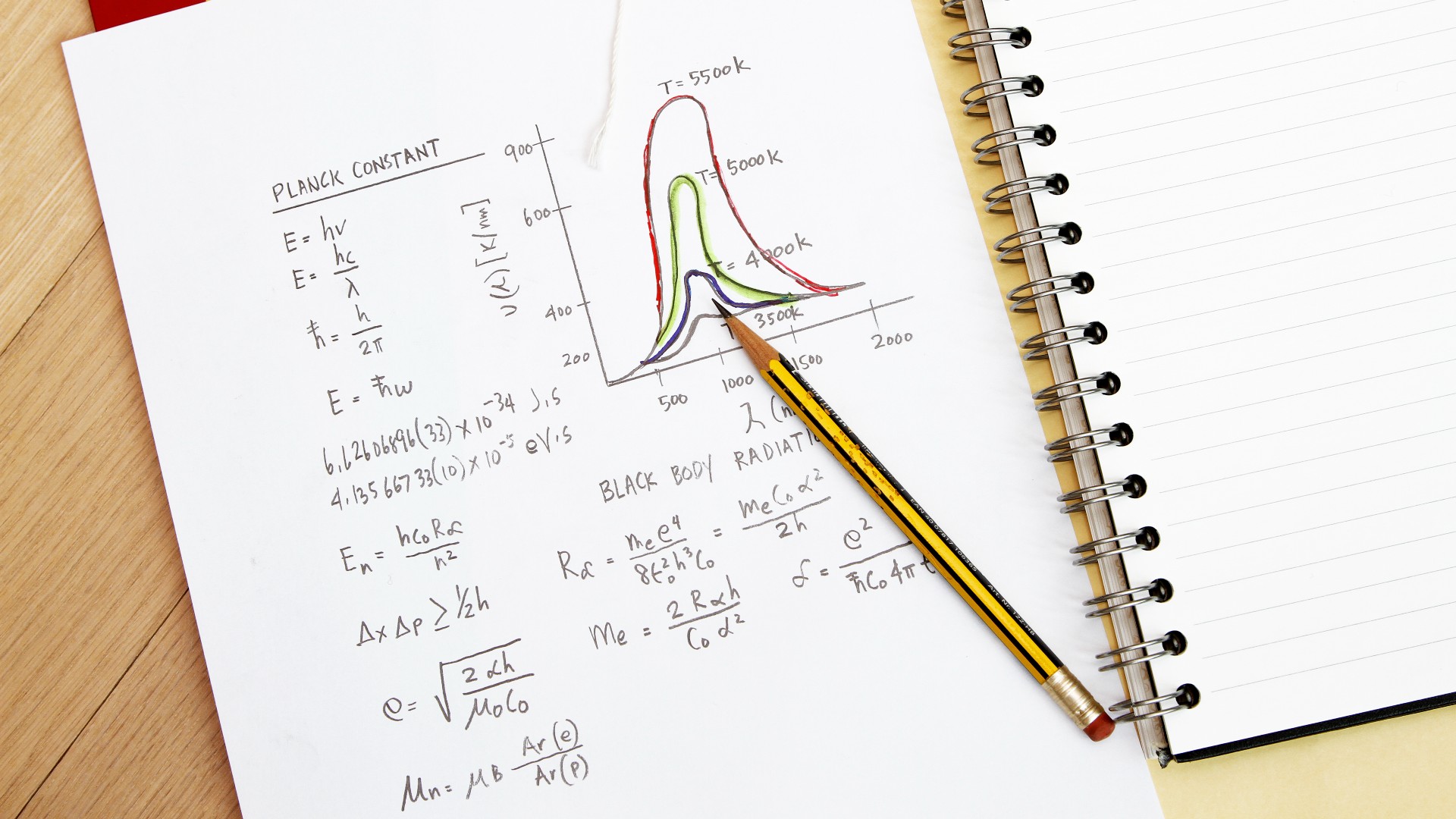
These ideas were not just pulled out of a hat. According to the HyperPhysics reference page at Georgia State University, German physicist Max Planck proposed that the emission of radiation might be quantized, meaning that an object could only absorb or emit radiation in chunks, and not have any value. The smallest of the chunks was a constant. Scientists used to think that emissions were continuous, meaning particles could travel at any given time.
The units of the constant are the same as the units of the object. The idea was imported from the idea that the smallest possible spin of an electron would be the same as the constant. The value could be twice or three times, but never any fraction of it.
It would take the full development of quantum mechanics to understand why electrons have a minimum and higher orbits. Particles and waves are the same. We can just as easily see an electron as a wave around the nucleus.
Waves have to obey rules. They need standing waves that fit inside the space to make them. When someone plays a musical instrument, only certain wavelength will fit, so you have separate notes. The first wave of an electron has to fit around a nucleus in order for an electron to travel to that location.
The basic point is that an electron can't get any closer to a nucleus because of its quantum mechanical nature.
There is a different way to look at a situation that doesn't rely on quantum mechanics. The nucleus is being pulled closer to the electron by it's electrical attraction. The electron has a mechanism to send it away.
These two are in balance. The total energy of an electron in the sky is negative. Adding energy to the atom is necessary to remove the electron. If you wanted to remove a planet from the solar system, you would need to add energy to the system.
One way to see this situation is to imagine an electron falling towards a nucleus. The rules of quantum mechanics prevent it from reaching the nucleus. It gets stuck for a long time. The total energy of the system is negative, which means it's stable and bound together, forming a long- lasting atom.
The original article was published on Live Science in 2011.