Two and a half years ago, a disease that later spread to the rest of the world was identified as the cause of an outbreak of a mysterious severe viral pneumonia in Wuhan, China. The most likely explanation was that the disease developed the ability to jump from an animal to a human. The hypothesis that the novel coronaviruses was created in a laboratory and then escaped through incompetence or malfeasance was far less plausible. Steve Novella has already talked about the studie s, but having a few days to look at the studies gave me the chance to look at the reaction to the studies. Contrary to the claims of some optimists, these studies haven't made much of an impact on conspiracy theorists, other than to provide them with targets to try to undermine them.
Let's take a look at the lab leak hypothesis and conspiracy theories first. This is done for two reasons. I want to show what these studies tell us about the origin of the disease. It has been a long time since I wrote about this issue.
Since the beginning of the Pandemic there has been a question of whether or not the disease came from a lab or not. The latter hypothesis didn't start out as a conspiracy theory, as lab leaks have happened before, although none had ever caused a Pandemic. It quickly took on the characteristics of a conspiracy theory, such that even those advocating the "lab leak" hypothesis had difficulty not interspersing more serious scientific arguments with conspiratorial thinking. The lab leak hypothesis continued to drift further and further from legitimate science and deeper into conspiracyland, making it hard to find examples of lab leak advocates who don't add conspiracy mongering narratives to their arguments.
This is what I mean. By May 2021, it had developed all the hallmarks of a conspiracy theory, including a coverup narrative in which China and powerful forces in the US were suppressing all mention of a lab leak as a conspiracy theory. It didn't help that the WIV was close to the wet markets that were first identified as the likely source of the outbreak and that they were studying coronaviruses. The lab leak proponents are fond of other types of conspiratorial thinking, such as weaponization of disagreements within a general consensus, shifting the burden of proof, and others, all with an intense belief in a cover.
Conspiracy theories about a new pathogen leaking from a biowarfare research lab are not new. Whenever a deadly new pathogen appears to cause a major outbreak or a global epidemic, they inevitably arise. It happened with several diseases. There was a major conspiracy theory about the creation of HIV/AIDS and how it was created at Fort Detrick. This was a Russian propaganda operation designed to blame the AIDS epidemic on the US. There are other conspiracy theories that claim that HIV got into the population by getting into the vaccines. Conspiracy theories about a lab origin for the virus, as well as other things, sprang up when it was learned that the initial outbreak in China was caused by the disease.
By February 2020, one of the earliest conspiracy theories had arisen, when James Lyons-Weiler claimed to have broken the coronaviruses code, and that the novel coronaviruses had been published a week or two before. He tried to claim that the novel coronaviruses had evidence of a plasmid, which is a circular DNA construct that scientists insert genes that can then be introduced into cells to get them to make the product of those genes. Lyons-Weiler made some rather glaring errors when he claimed to have expertise in the field ofmolecular biology. The claim that there were HIV sequences in the virus that indicated that there was a failed attempt to develop a vaccine against AIDS was one of several variations on the theme.
Scientists concluded that the virus was not likely to have been engineered in a laboratory after examining the sequence of the nucleotides. That revelation didn't stop the conspiracy theories that the virus had been created in a lab and escaped. It took a long time to accumulate enough evidence. There were distorted claims about the rarity of certain base combinations in the viruses. Steve noted that by late last year it had become clear that the narratives were not consistent with the data. The lab leak hypothesis was a lab leak, just of a naturally occurring coronaviruses, which made it harder to say that it was a bio weapon. There were claims that workers at WIV were exposed to the disease in November, but contact tracing couldn't find any cases.
This isn't saying that the lab leak hypothesis isn't possible. Lab leaks of pathogens have happened before, but they have not led to a global epidemic. Conspiracy theorists assumed that because lab leak was possible that it was equally likely as a natural origin, and that's what the evidence shows. I will quote Dan Samorodnitsky before moving on to the studies.
If the question is “are both hypotheses possible?” the answer is yes. Both are possible. If the question is “are they equally likely?” the answer is absolutely not. One hypothesis requires a colossal cover-up and the silent, unswerving, leak-proof compliance of a vast network of scientists, civilians, and government officials for over a year. The other requires only for biology to behave as it always has, for a family of viruses that have done this before to do it again. The zoonotic spillover hypothesis is simple and explains everything. It’s scientific malpractice to pretend that one idea is equally as meritorious as the other. The lab-leak hypothesis is a scientific deus ex machina, a narrative shortcut that points a finger at a specific set of bad actors. I would be embarrassed to stand up in front of a room of scientists, lay out both hypotheses, and then pretend that one isn’t clearly, obviously better than the other.
The two studies published last week add to the difference between lab leak and lab cover-up. There are a number of coronaviruses that are known to have an animal origin and that there is no data to suggest that the WIV or any other laboratory was working on the disease.
The first study came from the University of California at San Diego. The list of authors is long because studies of this sort require a lot of expertise and materials and single institutions rarely have everything needed to do them.
I can help to summarize the findings even though I don't know much about bioinformatics ormolecular biology, as I read the whole paper and the supplementary materials and figures. The authors queried several large sequence databases maintained by different countries, including the GISAID database, GenBank, and the National Genomics Data Center of the China National Center for Bioinformatics. They analyzed the sequence by computer to reconstruct the likely origin and evolution of different viral lineages and used epidemic modeling to surmise when the virus was likely introduced into the human population.
The first finding is that it's highly unlikely that the disease spread to humans before the year's end. The first zoonotic transfer from animals to humans is thought to have taken place around November 19, 2019. According to a news story, the earliest confirmed case of COVID-19 in China could be traced back to November 17 of last year. The first person to contract COVID-19 might have been a 55 year old man from Hubei province. One to five new infections were reported each day after that, and by December 15 there were 27 infections. The number of confirmed cases had reached 60 by December 20 and then it was off to the races for an outbreak.
The scientists have found that a single zoonotic event can't explain the data, but rather require two events from two different lineages. The jump to humans was made by Lineage B. It was only found in people who were connected to the market. It was only in samples from humans who lived near or stayed close to the market that it was discovered. The paper was from the front.
Therefore, our results indicate that lineage B was introduced into humans no earlier than late-October and likely in mid-November 2019, and the introduction of lineage A occurred within days to weeks of this event.
And.
The first zoonotic transmission likely involved lineage B viruses around 18 November 2019 (23 October–8 December), while the separate introduction of lineage A likely occurred within weeks of this event. These findings indicate that it is unlikely that SARS-CoV-2 circulated widely in humans prior to November 2019 and define the narrow window between when SARS-CoV-2 first jumped into humans and when the first cases of COVID-19 were reported. As with other coronaviruses, SARS-CoV-2 emergence likely resulted from multiple zoonotic events.
I am aware of what lab leak proponents are thinking. The novel coronaviruses were introduced into the human population. Isn't that very likely?
Yes and no, that's right.
The likelihood that such a virus would emerge from two different events is low, acknowledged co-author Joel Wertheim, an associate adjunct professor of medicine at the University of California, San Diego.
“Now, I realize it sounds like I just said that a once-in-a-generation event happened twice in short succession, and pandemics are indeed rare, but once all the conditions are in place — that is a zoonotic virus capable of both human infection and human transmission that is in close proximity to humans — the barriers to spillover have been lowered such that multiple introductions, we believe, should actually be expected,” Wertheim said.
The authors noted that as well.
Successful transmission of both lineage A and B viruses after independent zoonotic events indicates that evolutionary adaptation within humans was not needed for SARS-CoV-2 to spread (49). We now know that SARS-CoV-2 can readily spread after reverse-zoonosis to Syrian hamsters (Mesocricetus auratus), American mink (Neovison vison), and white-tailed deer (Odocoileus virginianus), indicating its host generalist capacity (50–55). Furthermore, once an animal virus acquires the capacity for human infection and transmission, the only remaining barrier to spillover is contact between humans and the pathogen. Thereafter, a single zoonotic transmission event indicates the conditions necessary for spillovers have been met, which portends additional jumps. For example, there were at least two zoonotic jumps of SARS-CoV-2 into humans from pet hamsters in Hong Kong (56) and dozens from minks to humans on Dutch fur farms (52, 53).
It shouldn't be surprising that there were more than one introduction to the disease. This study supports the idea that the most likely origin of the disease was in an animal and that it first appeared in humans in November of last year. The study didn't identify the animal. It is strong.
The second study, what about it?
There is a large list of authors from different institutions in the second study. There is a correlation between the first study and the emergence of COVID-19 in late 2019.
The authors looked at a lot of data.
COVID-19 case data from December 2019 was obtained from the WHO mission report (7) and our previous analyses (5). Location information was extracted and sensitivity analyses performed to confirm accuracy and assess potential ascertainment bias. Geotagged January/February 2020 data from Weibo COVID-19 help seekers was obtained from the authors (26). Population density data was obtained from worldpop.org (27). Sequencing- or qPCR-based environmental sample SARS-CoV-2 positivity from the Huanan market was obtained from a January 2020 China CDC report (data S1) (24).
Also, that's right.
To estimate the relative amount of intra-urban human traffic to the Huanan market compared to other locations within the city of Wuhan, we utilized a location-specific dataset of social media check-ins in the Sina Visitor System as shared by Li et al. 2015 (33). This dataset is based on 1,491,499 individual check-in events across the city of Wuhan from the years 2013-2014 (5-6 years before the start of the COVID-19 pandemic), and 770,521 visits are associated with 312,190 unique user identifiers. Location names and categories were translated using a Python API for Google Translate.
The investigators found what they were looking for. A picture is worth a thousand words.
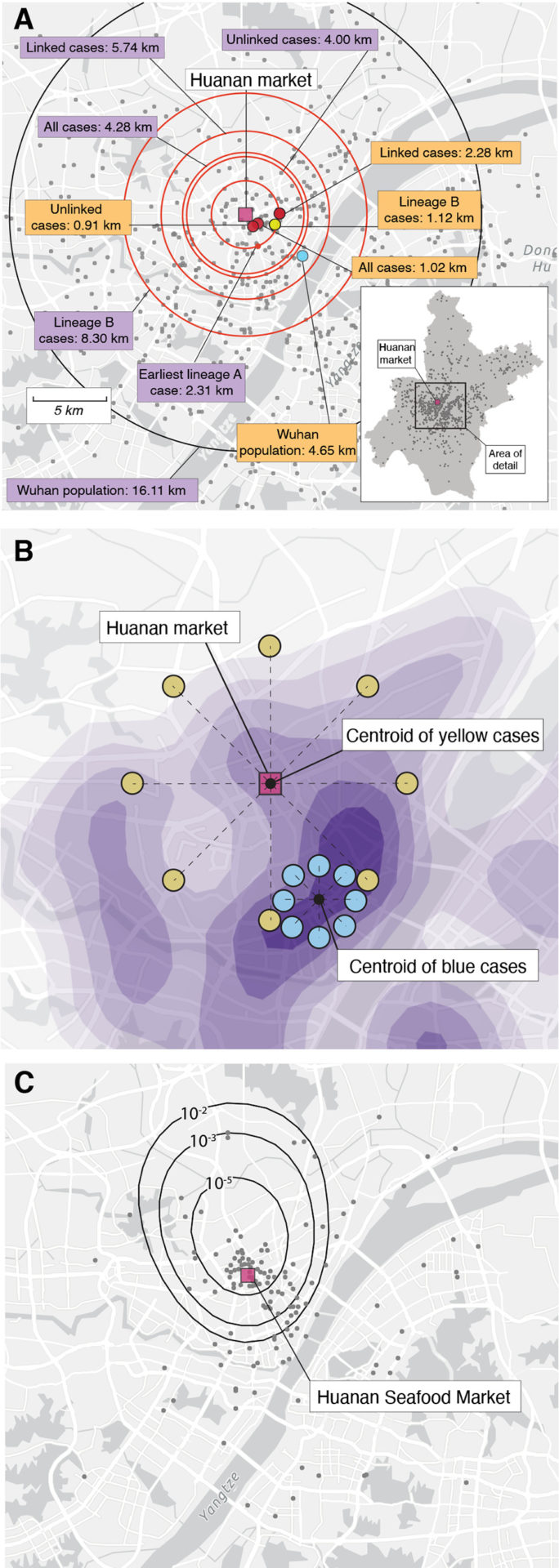
There is a second fig. Analysis of spatial data.
The map shows 1000 random samples from worldpop.com. The median distance between Huanan market and worldpop.org is shown in the main panel. The blue dot shows the center point of the data. The center-points of December cases are shown by red dots. There is a distance from the center-points to the market. The schematic shows how cases can be close to a specific location. If the epicenter of the epidemic is the Huanan market, then early cases should be centered on it. The blue cases show that the cases weren't centered on the market. The relative risk of COVID-19 cases in December is compared to the data from January to February. The locations are shown in the dots. If the December cases had been drawn from the same spatial distribution as the January-February data, the contours would show the density of December cases within bounds.