Every moment in our body's cells, there are many important activities that occur thanks to the enzymes. The special proteins act as catalysts by speeding the pace and improving the selectivity of chemical reactions. The food, pharmaceutical, agriculture, and cosmetics industries rely on enzymes for many processes.
Ignoring their importance and ubiquity, they are not understood. The pocket-like region where the sped up chemical reactions take place is what scientists want to know. While the three-dimensional atomic structures of many enzymes' active sites have been visualized and mapped, the invisible structure of the electric fields inside an active site is not. The electric fields are thought to play an important role in forming a precise environment in active sites.
A new probe for measuring and seeing the electric fields inside an enzyme's active site has been unveiled by a study co-led by researchers from the University of California, Berkeley. The orientation of electric fields at the site of the reaction is reported in a recent paper. These insights could lead to the creation of custom-tailored synthetic enzymes for industry as well as the advancement of the discovery and design of new drugs that interfere with or modify the function of enzyme targets.
A graduate student in the lab of Steven G. Boxer said that they have developed a novel probe that can give them important information about how electric fields are unique to enzymes.
"On a basic level, we are trying to better understand how enzymes work, and in this study, we are adding a new dimension by bringing in electric field orientations which is believed to have a critical impact on enzyme's catalytic functions."
A powerful tool.
The Boxer lab atSTANFORD has pioneered the idea of interpreting the function of enzymes by measuring electrostatic interactions, which are present in all forms of matter and are specifically organized in three dimensions.
Boxer said that the question of the origin of the amazing function of enzymes is a general one. 80% of all chemicals are made using catalysts, but what is actually responsible for lowering the activation free energy is not understood for most reactions. Boxer is a senior co-author of the study and the chair of the Department of Chemistry at the school of humanities and sciences.
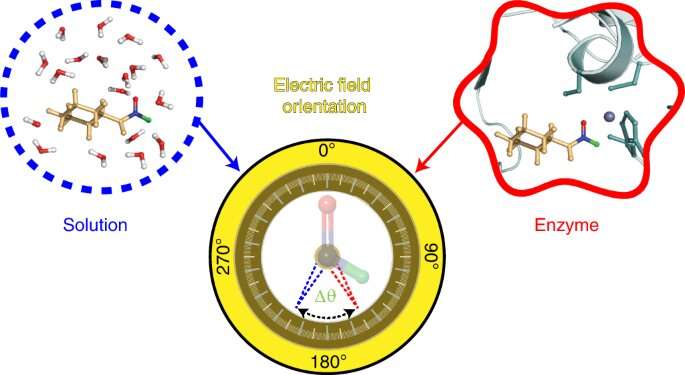
The technique used to develop the probe was developed in the Boxer lab. The electric fields are revealed by the shifts in these frequencies. The researchers used a probe made from a molecule called N-cyclohexylformamide. The molecule binding to the active site of the alcohol dehydrogenase is an idiosyncrasy.
The researchers were able to see the electric field in the active site of the alcohol dehydrogenase by targeting two bonds in the probe. The angle between the bonds allowed the researchers to gauge not only the strength of the field, but also its orientation. The magnitude of electric fields was not reported in previous studies from the Boxer lab.
The tool is called a two-direction probe because it allows us to measure the electric field in two different ways. The orientation information about the electric field can be reconstructed using this method. It hasn't been done before.
The chemical sleight of hand was used to gather the key measurement. It's hard to see the chemical bonds between a carbon atom and a hydrogen atom. The researchers swapped the hydrogen atom for a heavier one. Researchers were able to reveal the orientation of the electric field with the help of the new carbon–deuterium bond.
The environment is precise.
The electric field's interactions with N-cyclohexylformamide, modified with deuterium, were described by the researchers. The electric fields found in water, acetone, and other common solvent were compared to those found in those properties.
The result supports the idea of a preorganized electrostatic environment, or one in which the precise positioning of amino acids and the electrostatic environment they create helps reduce the energy required for a chemical reaction to take place. This could be a key to the success of the enzymes.
Mao said that the study will help to advance the concept of correlation between the performance of the enzymes and the electric fields. Evidence that electric fields in the active sites of the enzymes are preorganized is an important clue to the mystery of why they have their amazing abilities.
The probe could be used to investigate other active sites. Scientists and engineers will be able to design custom enzymes with new characteristics if knowledge is deepened.
The ultimate goal of this research is to enable us to design enzymes that have great performance in both industrial and medical applications. We are not there yet, but we are making progress and have a better understanding of how enzymes work.
More information: Chu Zheng et al, A two-directional vibrational probe reveals different electric field orientations in solution and an enzyme active site, Nature Chemistry (2022). DOI: 10.1038/s41557-022-00937-w Journal information: Nature Chemistry