Rice University chemical engineers have improved their design for a light-powered catalyst that quickly breaks down PFOA, one of the world's most problematic pollutants.
Michael Wong and his students discovered in 2020 that a commercially available powder that is commonly used in cosmetics can destroy 99% of PFOA in water samples within a few hours.
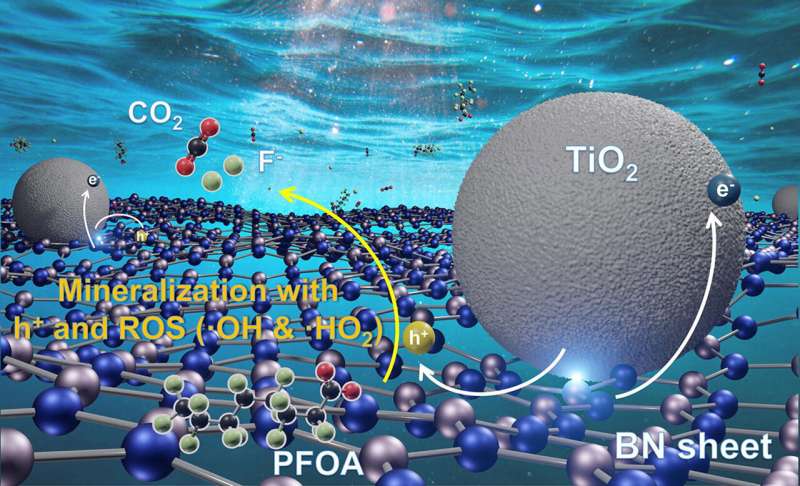
Wong is the author of a study about the redesign of the catalyst in the Chemical Engineering Journal. It was less than ideal due to the fact that the atmosphere filters out most of the short-wave UV from the sun. We wanted to increase the amount of energy that can be accessed from other wavelength of sunlight.
The wavelength of long-wave UV is about 400 nanometers. Suntans and sunburns are caused by it, and it's plentiful in sunlight that reaches Earth. Boron nitride isn't activated by UV- A. Titanium dioxide, a common ingredient in sunscreen, has been shown to break down PFOA, albeit slowly, when exposed to UV- A.
The best features of the individual catalysts were taken into account by Wong and his co-lead authors. The UV-A powered composites destroyed PFOA 15 times faster than titanium dioxide photocatalysts.
Wong's team learned how to break apart PFOA by analyzing photocurrent response measurement and other data. In outdoor experiments using plastic water bottles under natural sunlight, they found the boron nitride-titanium dioxide composites could degrade about 99% of PFOA in less than 3 hours. It took about nine hours for that process to take place in salty water.
There is mounting evidence that PFOA is harmful to humans. The EPA plans to develop federal standards for PFOA in drinking water in March 2021.
Water treatment plants are looking for new and cost-effective ways to remove PFOA from water because of growing regulatory pressure.
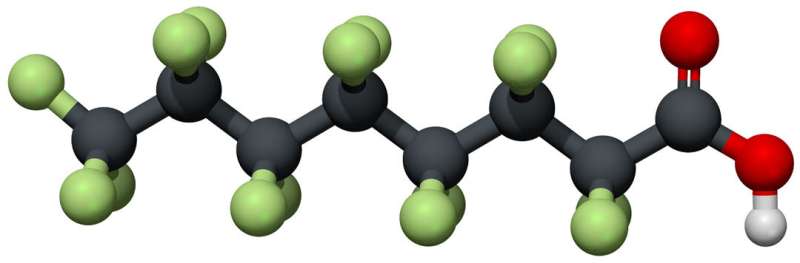
A family of compounds developed in the 20th century to make coating for waterproof clothing, food packaging and other products is known as PFOA. The term "forever chemicals" was used because they aren't easy to degrade and linger in the environment. Wong's team is looking at how well the photocatalyst works.
Several industrial partners in the Rice-based Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (NewT), which is funded by the National Science Foundation to develop off-grid water treatment systems, are interested in the Boron nitride and composite catalyst technologies.
More information: Lijie Duan et al, Titanium oxide improves boron nitride photocatalytic degradation of perfluorooctanoic acid, Chemical Engineering Journal (2022). DOI: 10.1016/j.cej.2022.137735