According to a study published in the Proceedings of the National Academy of Sciences, investigators have found novel mechanisms that regulate a cell's response to stress.
Cancer and other diseases use stress response programming to survive, according to the findings.
These deranged genomes are found in cancer. The senior author of the study said that the stress response genes are critical for cancer survival.
The lead author of the study was a student in the MSTP.
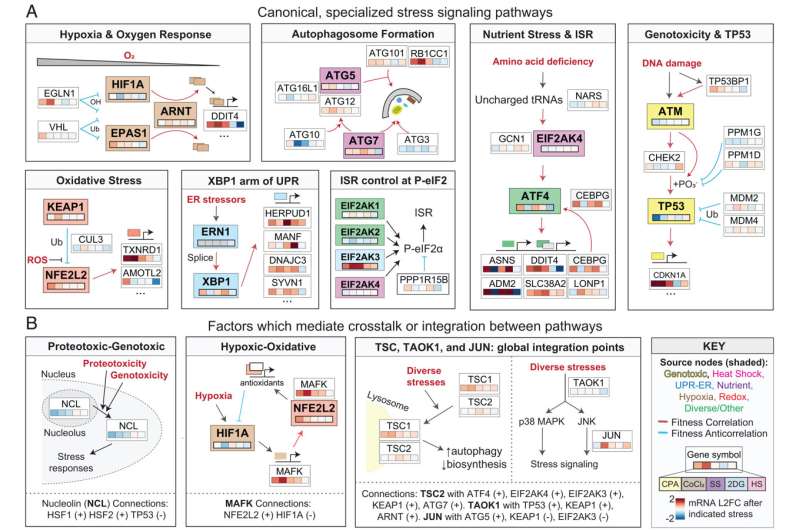
Human cells are able to counteract different types of stressors. The mechanisms which help regulate and link these stress response pathways have remained poorly understood because they are studied individually.
In the current study, Mendillo's team used genome-scale fitness screening data compiled by a computational tool called FIREWORKS, which was developed by Mendillo's laboratory, to map the stress phenotypes of more than 700 cancer cell lines
The approach showed a network of genes that promote stress responses.
They found that HAPSTR1 plays a central role in cellular stress signaling and is also caused by certain types of stressors.
HAPSTR1 interacts with and is degraded by a molecule called HUWE1, which has been implicated in a number of diseases. The sole function of the E3 ubiquitin ligase is to hunt down and degrade specificProteins
The relationship between HUWE1 and HAPSTR1 is atypical, as HUWE1 feeds back to promote the degradation of HAPSTR1.
The relationship is complicated by the fact that turning off either one results in the same effects.
The findings show that HAPSTR1 is a central player in a network of stress response pathways that promote cellular adaptation.
We know that HAPSTR1 promotes cellular resilience to these types of stresses. This is going to be important in cancer and other diseases.
More information: David R. Amici et al, C16orf72/HAPSTR1 is a molecular rheostat in an integrated network of stress response pathways, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2111262119 Journal information: Proceedings of the National Academy of Sciences