There's a chance that paramyxoviruses could cause a devastating epidemic. Measles is one of the Viruses in this family.
Measles is the most infectious of all knownviruses. According to Michael Norris, assistant professor at the University of Toronto, if one person coughs in a room with 100 unvaccinated people, around 90 will get the disease. The Nipah virus causes between 40% and 90% of deaths.
Imagine if a paramyxoviruses emerged that was as deadly as the Nipahviruses.
It's easy to see that scenario. The movie "Contagion" was based on an imagined paramyxoviruses.
This is the first-ever look at a key stage in the life cycle of a disease. Future therapies may be able to stop the viruses in their tracks according to a new study.
The work solved a long-standing mystery about how viruses assemble themselves. We know that a virus's many pieces come together at the cell, but we don't know what triggered the assembly process.
The study shows how paramyxoviruses can use a host cell to spread their infections. Drug discovery endeavors will be informed by this work.
There is a paramyxoviruses.
X-ray crystallography and electron microscopy were used by the researchers.
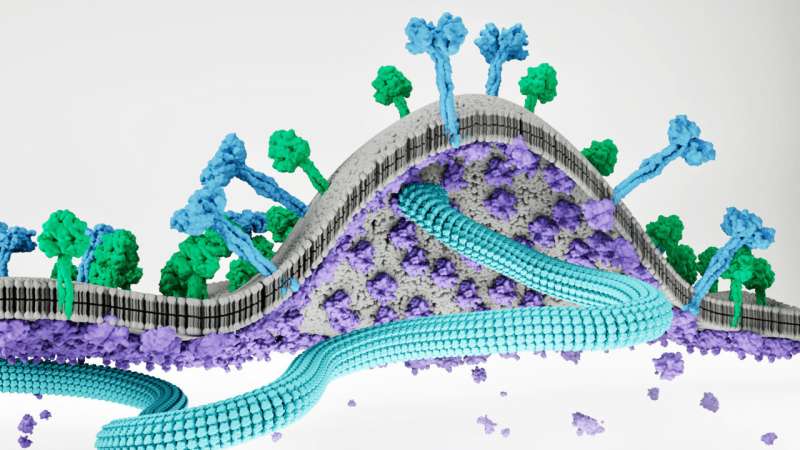
Key genes and genetic material rush to certain areas on the host cell membranes. The matrix proteins form a lattice against the inside of the cell. The drivers of the virus assembly process are matrix genes. The field marshals gather and guide the other components of a new virus. A Viruses shape is also given by matrix proteins.
As viral assembly continues, the lattice of matrix proteins begins to push the membranes outward to form a "bud" and recruit other viral proteins to this site. Once the bud has all the necessary components in place, it splits away from the parent cell to form a new virus that can then spread to other cells.
Researchers want to design therapies that interrupt the process of viral assembly. The drug is in clinical trials and could be used to stop paramyxoviruses. The therapy is a proof of principle that targeting viral assembly is a viable strategy for drug development.
A clear view of the paramyxoviruses assembly process is needed by scientists. Researchers are trying to see the action of the matrix.
Capturing a group of people.
They looked at the assembly of the measles and Nipahviruses. A two-sided "dimer" structure is formed by two matrix proteins coming together in a kind of hug. It wasn't surprising that the researchers showed that stopping thishug by blocking formation of this dimer stopped viral assembly.
The researchers wanted to know how the dimers interact with other structures. They showed that the hug floats towards the inside of the cell's outer shell.
The team found that the matrix proteins bind to a lipid molecule in the host cell. The interaction provides meeting points for virus assembly on the host cell's surface. They used X-ray crystallography to capture this interaction.
There was a big structural shock. The team found that the Nipah virus matrix changes its structure to open up a pocket of lipids.
This was an exciting part of the study. The pocket would not have been seen without the structure captured in the study. There is a brand-new target discovered in this pocket.
The secret ingredient that leads to the formation of a lattice on the inner surface of the cell is called PI(4,5)P 2. When the matrix proteins change their structure, they adopt a shape that drives lattice assembly.
The bend in the cell membranes is a result of this change in the matrix. The space between two matrix proteins in a hug is shaped like a bowl. The bowl shape is transformed into a plate shape by the sides flattening after the binding of PI(4,5)P 2. A new bud that will eventually make up a new virus is formed by the soft curve of the cell membranes.
These structures taught the scientists a lot. As the process was triggered, we didn't know how much the molecule would change or what the structure would look like.
A broad therapy is being designed.
Around the world, measles is still a big problem. Each year, India and Bangladesh deal with an outbreak of the NIPah. We need effective therapies to stop the spread of these Viruses.
Animals could be protected from disease and food security could be ensured. Paramyxoviruses can wipe out entire flocks before symptoms even show up. In California, 1.2 million birds were culled due to an outbreak of disease.
A new study shows the potential for a therapy that targets multipleviruses. The genomes of the two viruses are very different, but the same matrix proteins.
"Because these matrix structures are highly conserved, we could potentially target one virus and have an inhibitor that could target all the viruses in this family."
Thanks to the funding from the Tullie and Rickey Families SPARK Award for Innovation in Immunology, Norris was able to start looking for matrixproteins. Over 7.4 million drug candidates were narrowed down to around 100 that will be tested.
The next steps are to better understand the interactions that make up the matrix lattice and to better understand how matrix proteins interact with each other.
We're looking at using this work to design broad-spectrum inhibitors of viral assembly
More information: Michael Norris et al, Measles and Nipah virus assembly: specific lipid binding drives matrix polymerization, Science Advances (2022). DOI: 10.1126/sciadv.abn1440. www.science.org/doi/10.1126/sciadv.abn1440 Journal information: Science Advances