Rice University scientists who flash materials to synthesise substances like graphene have turned their attention to a material that is highly valued for its thermal and chemical stability.
The process by the Rice lab of chemist James Tour shows a way to quickly heat and cool to produce two-dimensional materials. It was nearly impossible to make both in bulk and in easily usable form.
The lab's report shows how flash Joule heating, a technique introduced by the Tour lab in 2020, can be used to prepare a variety of materials.
Experiments with the material showed it could be used as a coating.
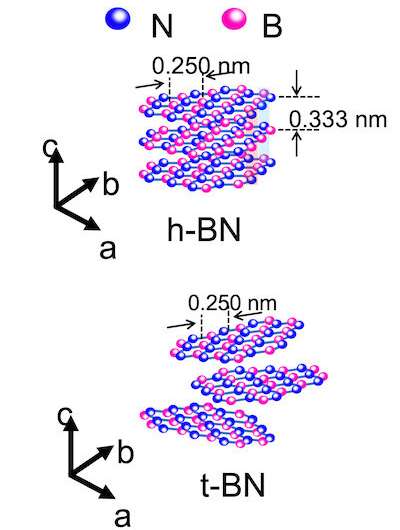
Boron nitride is a very sought after material. It's even more versatile because of the fact that it can be made in bulk and with mixed amounts of carbon.
A hexagonal configuration with alternating boron and nitrogen atoms is a form of boron nitride. Boron nitride is soft, so it's often used as a lubricant and as an enhancer to cosmetics, as well as being found in ceramics and metal compounds to improve their ability to handle high heat.
Michael Wong, a Rice chemical engineer, reported that Boron nitride is an effective catalyst in helping to destroy a dangerous chemical found in the environment and in humans.
The method of flash joule heating involves stuffing source materials between two tubes and sending a small electrical current through them. Food waste and used plastic car parts are just two examples of things that can be made out of Graphene. The process has isolated rare earth elements from other sources.
The lab fed ammonia borane into the flash chamber with different amounts of carbon black for different purposes. The sample was flashed two times, first with 200 volts to degas the sample of extraneous elements and again with 150 volts to complete the process, with a total flashing time of less than a second.
The microscope images showed that the flakes are not evenly distributed. It's easy to separate the flakes.
The anti-corrosion experiments took place because they're easilysoluble. The lab painted the compound on copper film and exposed it to a bath of acid.
The flashed compound was more effective at protecting the copper than any other compound. The compound created "tortuous pathways for corrosive electrolytes" to reach the copper, as well as preventing metal ion from moving.
Adding carbon can be used to adjust the conductivity of the precursor.
The lab is interested in flashing additional materials. "Precursors that have been used in other methods can be tried in our flash method to see if we can prepare more products with stable features," Chen said. There is more research to be done on flashing metastable phase metal carbides and transition metal dichalcogenide.
More information: Weiyin Chen et al, Turbostratic Boron‐Carbon‐Nitrogen and Boron‐Nitride by Flash Joule Heating, Advanced Materials (2022). DOI: 10.1002/adma.202202666 Journal information: Advanced Materials