Cancer, genetic diseases, and as the world has learned in recent years, deadly viruses, are some of the diseases that can be treated with the use of messengerRNA.
If the drugs don't reach the right cell types, they won't work.
A team of researchers at the Georgia Institute of Technology and the School of Medicine have taken another step towards improving the development of custom-made delivery vehicles. The system developed by Dobrowolski and Paunovska makes pre-clinical studies more accurate. The direction of research is being influenced by their discoveries.
The McCamish Foundation Early Career Professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech expects to shift most of his future projects to this method.
The events are grouped together.
For the past few years, the two of them have worked together in a research enterprise. Both Santangelo's and Dahlman's labs use LNPs to deliver therapies.
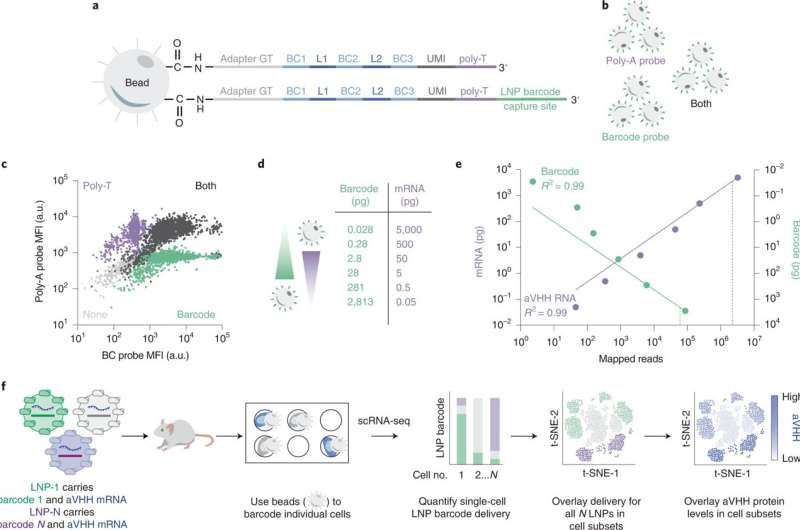
A technique called DNA barcoding has been developed by the team to speed up the testing of their LNPs. Researchers insert a piece of DNA that matches the LNP. After injecting the LNPs, cells are examined for the presence of the "barcodes". The most promising nanoparticles are highlighted by the system. The barcoding process allows many experiments to be performed at the same time.
The pre-clinical screening process has been improved by the use of DNA bar coding. There is still a problem with drug delivery. Cells are like moving targets because they are diverse. Cells that were thought to be homogeneity are actually composed of different cell subsets. The team surmised that the chemical and genetic heterogeneity has a powerful influence on how well LNPs can deliver.
"Cells are complex, and they don't have a single one that defines them." If we're being honest, they are best defined using all the genes they do, or do not, express.
The researchers created a tool to measure all of these things at once. They have a single-cell nanoparticle targeting-sequencing system.
The approach is called multiomics.
The researchers were able to quantify how LNPs deliver barcodes and mRNA into cells, as well as the identity of the cell in thousands of individual cells.
It could be an important step forward for high-throughput LNP discovery. The team was able to identify the cell types that show high or low nanoparticles use with the help of the SENT-seq technique.
In addition to testing the efficacy of a drug and how certain cell types respond to it, they're also identifying which genes are involved in successful LNPs absorption. They are doing it all at the same time.
The data shows that different cell subsets have different responses to nanoparticles. There is still a lot of work to be done, but we believe the ability to simultaneously read out high-throughput nanoparticle delivery and the cellular response to nanoparticles will lead to better mRNA therapies.
After two months of working together, co-lead authors Paunovska and Dobrowolski came up with the idea for the SENT-seq system.
The work that was done in the lab was done by the team. This is the start of an interesting phase in our work.
More information: Curtis Dobrowolski et al, Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery, Nature Nanotechnology (2022). DOI: 10.1038/s41565-022-01146-9 Journal information: Nature Nanotechnology