An international team of researchers, led by scientists at the University of Manchester, has developed a fast and economical method of converting methane, or natural gas, into liquid methanol. The method uses visible light to drive the conversion.
The researchers used the VISION instrument at Oak Ridge National Laboratory to observe the process.
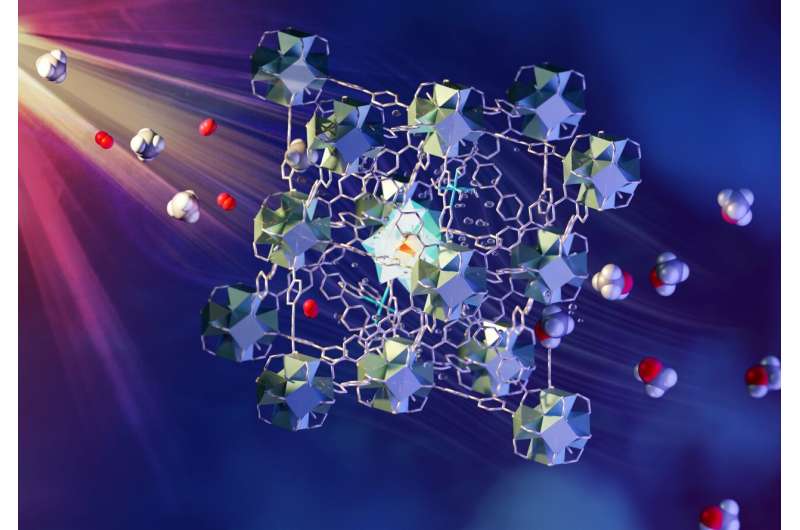
A novel metal-organic framework catalyst is used in the method. Each component of the MOF has a role to play in absorbing light, transferring electrons, and bringing together methane and oxygen. Methanol is easy to extract from the water. The US Department of Energy supports research in this area. The details of the team's findings are in Nature Materials.
Methane is an abundant and valuable fuel, used for ovens, furnaces, water heating, kilns, automobiles and turbine. Methane can be difficult to extract, transport and store.
When methane is released or leaked into the atmosphere, it is harmful to the environment. Fossil fuel production is one of the leading sources of atmospheric methane.
Excess methane can be burned off to reduce its impact on the environment. Carbon dioxide is a greenhouse gas.
Methane is a very popular and versatile feedstock used to make a variety of consumer and industrial products. The economic incentive to reduce methane emissions would be provided by this.
Methanol is a liquid that can be easily transported. It can be used to make thousands of products, including paints, glues, and chemicals. As petroleum reserves dwindle, the conversion of methane into a high value fuel such as methanol is more attractive.
The bond needs to be broken.
The challenge of breaking the C-H chemical bond is one of the main challenges of converting methane to methanol. Conventional methane conversion methods typically involve two stages, steam reforming and syngas oxidation, which are energy intensive, costly and inefficient.
A multicomponent MOF material and visible light are used in the methane-to-methanol process. While exposed to the light, a flow of CH 4 and O 2 saturated water is passed through a layer. The components are held in fixed positions within the porous superstructure. They work together to absorb light in order to create electrons which are passed to oxygen and methane inside the pores.
The process is 100%selective, meaning there is no undesirable by-product, comparable with methane monooxygenase.
Experiments show that the solid catalyst can be isolated, washed, dried and re-used for at least 10 cycles, or 200 hours of reaction time.
Plants convert light energy to chemical energy during photosynthesis. Plants absorb the sun and carbon dioxide. The elements are then converted into sugars, oxygen and water.
The process has been referred to as the holy grail of chemistry. Martin Schrder, vice president and dean of the faculty of science and engineering at Manchester, said that it may now be possible to convert the gas directly to methanol, a high- value chemical that can be used to produce fuels, pesticides, and fuel Additives for vehicles. The new material may be able to facilitate other types of chemical reactions, by serving as a sort of test tube in which we can combine different substances to see how they react.
The process can be seen using neutrons.
The VISION instrument was used to confirm the strong interactions between CH 4 and the mono- iron-hydroxyl sites in the MOF.
Anibal "Timmy" Ramirez Cuesta is the leader of the Chemical Spectroscopy Group at SNS. Strong and characteristic neutron scattering signals from their rotation and vibration, which are also sensitive to the local environment, are produced by methane molecule. This allows us to show the bond-weakening interactions between CH 4 and the MOF.
It's fast and economical.
By eliminating the need for high temperatures or pressures, and using the energy from sunlight to drive the photo-oxidation process, the new conversion method could save equipment and operating costs. The development of in-line processing that reduces costs will be aided by the higher speed of the process.
More information: Sihai Yang, Direct photo-oxidation of methane to methanol over a mono-iron hydroxyl site, Nature Materials (2022). DOI: 10.1038/s41563-022-01279-1. www.nature.com/articles/s41563-022-01279-1 Journal information: Nature Materials