Researchers working with Jonathan Barnes, assistant professor in the Department of Chemistry, have recently shown how molecule with interlocking ring architectures can be functionalized and incorporated into three-dimensional materials. The new work was led by Mark Nosiglia, a graduate student in Barnes' lab. The results were published in a journal.
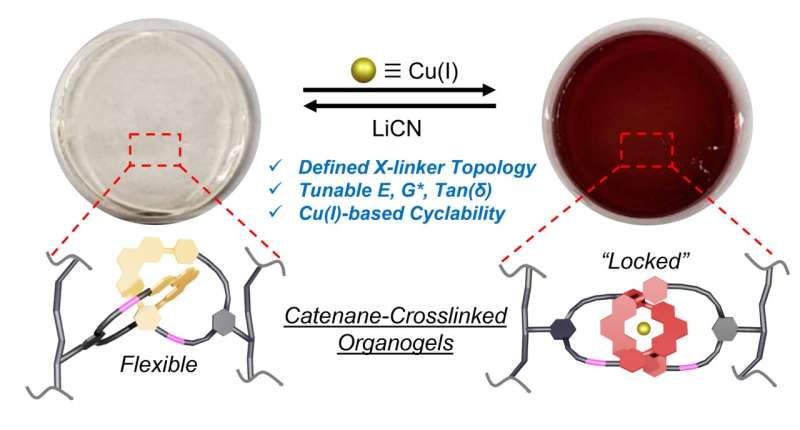
Barnes and Nosiglia integrated catenane-based crosslinkers into the network that makes up the material after streamlining and improving the efficiency of their method. When subjected to external forces, conjugates have enough freedom of movement to do things such as stretch and compress.
The gel material became more rigid and less elastic when Barnes and Nosiglia added a metal to the catenane.
Barnes said that it should be possible to tune the properties of materials by incorporating chain locks. It is possible to use the molecular ring architectures in rubber-like materials and plastics to improve stretchability and their ability to dissipate forces.
To explore and test their mechanical properties, Barnes, Nosiglia, and their partners are focusing on the production of their 3D networked materials at large scales. The team's future research endeavors will depend on scaling up.
More information: Mark A. Nosiglia et al, Metalation/Demetalation as a Postgelation Strategy To Tune the Mechanical Properties of Catenane-Crosslinked Gels, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c03166 Journal information: Journal of the American Chemical Society