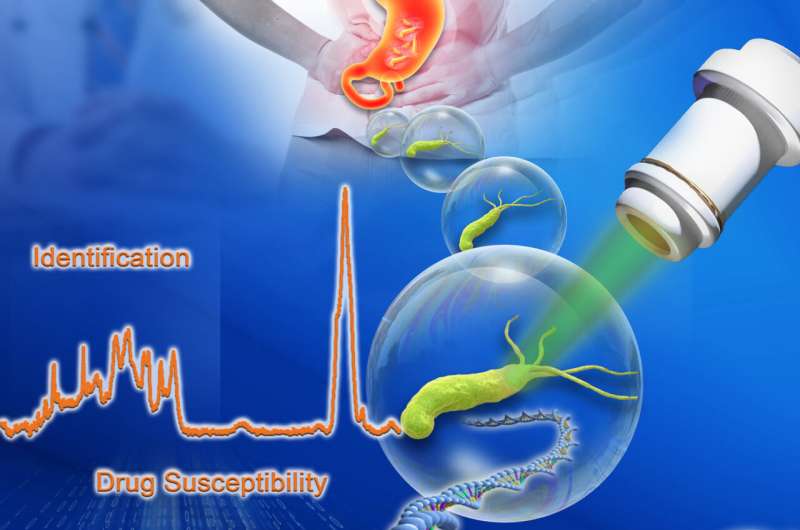
About half of the world's population is colonized by Helicobacter pylori, abacteria that can cause diseases. Selecting the right combination of antibiotics is important. Current tools are limited due to the slow growth of H. pylori.
The researchers from the Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences collaborated with the State Key Laboratory of Infectious Disease Prevention and Control.
Their findings were published in a chemistry journal.
Researchers and health care professionals need tools that are fast, reliable and sensitive for pathogen identification and antimicrobial susceptibility tests.
Drug sensitivity testing is one of the methods used to detect H. pylori.
A senior author of the study said that the current culture-based antimicrobial susceptibility testing is too slow and takes at least a week to complete.
The team has come up with a way to perform rapid pathogen identification, metabolism inhibition-based antimicrobial susceptibility tests, and high-quality single-cell whole-genome Sequencing. Their approach provides more than 98% accuracy and is successful at one-cell resolution.
The CAST-R-HP instrument has the core technologies of D2O-probed ramanometry andRACS-Seq.
The culture-independency, speed, high resolution and comprehensive information output suggest CAST-R-HP as a powerful tool for diagnosis and treatment of H.
The team will explore ways to further accelerate the CAST-R- HP by developing a chip that can enrich the trace number of cells directly from the H. The time it takes for the test to come back could be reduced from three days to less than 24 hours.
The first author of the paper said that their next step would be to fully assess the utility of the workflow for all the first- and second-line antibiotics.
The CAST-R-HP can be used to map H. pylori heterogeneity. The risk of drug resistance in the general human population should be reduced by enabling identification, drug susceptibility tests, and whole-genome-based source tracking at single-cell resolution.
More information: Min Liu et al, Single-Cell Identification, Drug Susceptibility Test, and Whole-genome Sequencing of Helicobacter pylori Directly from Gastric Biopsy by Clinical Antimicrobial Susceptibility Test Ramanometry, Clinical Chemistry (2022). DOI: 10.1093/clinchem/hvac082 Journal information: Clinical Chemistry