Scientists have been researching for two years to find a cure for COVID-19. As the clinical trials needed to test them become more difficult, researchers fear that the development of new treatments could be jeopardized.
Vaccinations have led to a decline in the severity of disease. The existence of potent treatments is making it more difficult to conduct statistical analysis.
It was simpler to do research in the past. You have to design a study that meets the standards of care doctors want to provide. Elizabeth Hohmann is an infectious-disease expert at MassachusettsGeneral Hospital in Boston.
The World Health Organization and the US Food and Drug Administration recommend a number of different types of therapy for people with COVID-19. Steroids are among them. The risk of death was reduced for those already in the hospital. The chances of having to be hospitalized are lowered by others. Some countries that are fortunate enough to have access to these treatments have seen their death rates drop.
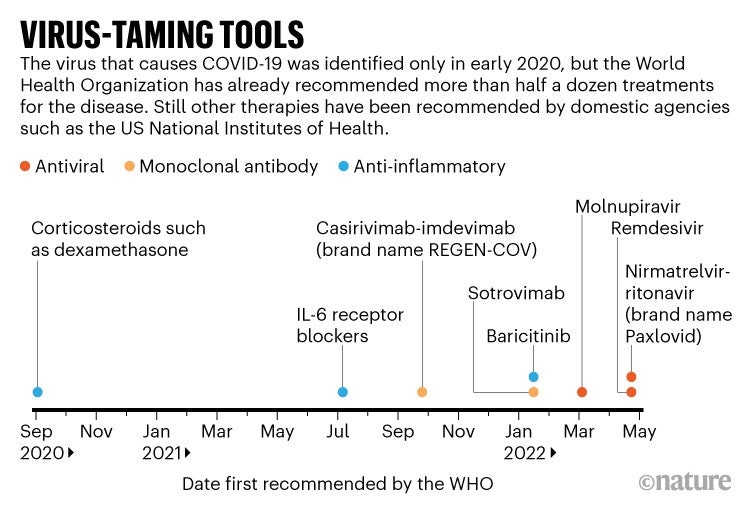
In many areas, the availability of therapies is limited and expensive. Paxlovid, developed by Pfizer in New York City, is a drug that could face resistance in the future. Even though many parts of the world are without treatment options, researchers worry that progress in establishing new treatments will stall.
Some hard-hit countries have seen death rates plummet due to vaccine use. The number of deaths in Brazil has fallen to less than 200 a day. That welcome news can make a trial more complicated.
Edward Mills and his colleagues set up a trial in Brazil to find out if existing drugs could prevent the most serious consequences of COVID-19. The share of study participants who died or had to be hospitalized was underestimated when the trial was launched. The number dropped after the vaccine became available. The organizers had to enroll more people who were in danger of dying in order to continue the testing. The trial had to be expanded to other places, such as South Africa, Pakistan, and the Democratic Republic of the Congo.
Scientists worry that people who qualify for trials are less willing to participate than they would have been at the beginning of the epidemic.
ill people had no better option than to enroll in the trial when Hohmann started overseeing it. The trial had more than one thousand participants by April 2020. By the end of 2020 it had been proven that the drug remdesivir can prevent death.
It became more and more difficult to find participants for future trials as effective treatments became available. Many people feel safer sticking with the established regimen than trying an experimental drug.
It takes a more adventurous person to take that third drug. If your life isn't at risk, it takes some civic-mindedness to sign up for a trial.
You can sign up for Scientific American's newsletters.
A Canadian clinical trial for the drug losartan might have been affected by hesitance. Most of the people available to join the losartan trial were unvaccinated due to the fact that most of the people in Canada became vaccine free. There was a rise in the number of people who were invited to join the study but did not. The same factors that make people hesitant to receive vaccines also make them less likely to take experimental drugs, according to trial organizers.
The complexity of the statistical calculations needed to determine the effectiveness of a new drug has increased as treatments have increased. It is possible that researchers will need to recruit more trial participants.
The organizers of the ongoing PRINCIPLE trial, which tests whether repurposed drugs can speed recovery or keep UK residents out of the hospital, have gone through a rough patch. All study participants receive the current standard of care, which means that doctors are free to prescribe treatments in addition to the drug being tested There is no difference in outcome between those taking placebo and those taking the treatment under study. Smaller differences mean that researchers need to work with bigger groups of people.
If researchers want to compare a new, highly effective drug against Paxlovid, the powerful antiviral that is now the leading treatment for early COVID-19, they will need to recruit a lot of trial participants. She says it would have to be a real game-changer to take on Paxlovid.
Finding a Paxlovid challenger can be difficult. Recent experiments on cells suggest that Paxlovid-resistant strains of the virus might arise, which is a stark reminder that the virus is defining the rules of the game.
The article was published on June 13th, 2022.