Researchers use techniques such as freezing plant samples or placing them in a vacuum to study the structure of plant cells in order to improve the quality of biomaterials. Valuable data can be provided but can also cause permanent damage to the samples.
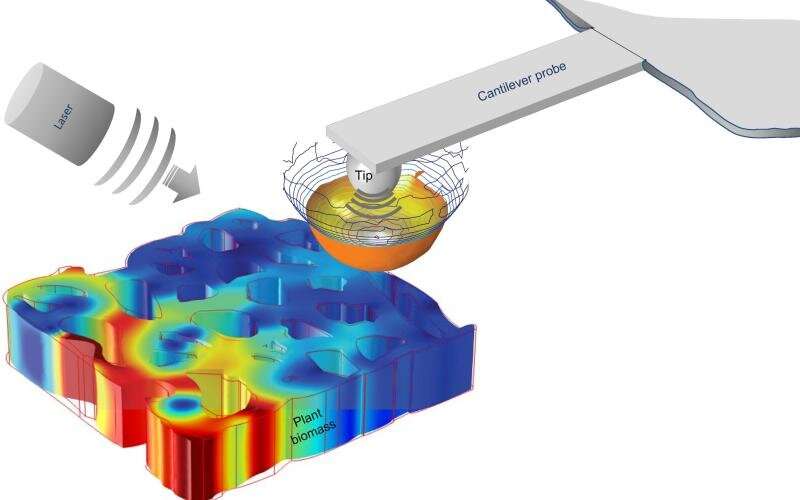
A team of physicists including Ali Passian, a research scientist at the Department of Energy's Oak Ridge National Laboratory, and researchers from the French National Centre for Scientific Research used state-of-the-art microscopy and Spectroscopy methods to provide nondestructive alternatives The team used a technique called scattering-type scanning near-field optical microscopy to examine the composition of cell walls from young poplar trees.
The team had other challenges to overcome. Passian describes the plant cell walls as a maze of intertwined spaghetti strings that are difficult to navigate. The researchers were able to see the optical properties of plant cell materials for the first time across regions large and small. The results were published in a magazine.
Passian said that the technique allowed them to look at the sample's optical and chemical properties at the same scale.
Along with ORNL and CNRS, the team included researchers from Aix-Marseille University and several other institutions.
Aude Lereu, a researcher at the Fresnel Institute, said that until now, these optical properties were not measured in situ but only from extracts.
The team was able to obtain a series of detailed images in one region of the poplar wood cell wall by using their measurement technique.
This data could be used to improve chemical treatments that use acids to increase yields and prevent biomaterials from degrading when exposed to outside factors. The researchers were able to identify both harmless and potentially harmful changes due to the delignification process that had already taken place.
Passian said that it's important to monitor how a material changes. We were able to study the sample while keeping a record of any changes that might affect it.
The technique used on poplar could provide similar data on other plants, which could be used to improve the efficiency of treatments and engineer ideal biomaterials.
Lereu said that their technique revealed that some types of lignin were not completely removed during delignification.
Additive manufacturing, which involves stacking layers of materials to create a wide variety of objects, could benefit from the technique. During the printing process, Passian describes as a more complex version of piping frosting onto a cake with a pastry bag, the measurement technique could add a layer of quality control to minimize human errors.
Gaining a front row seat to subtle changes in plant cells was a challenge, but Passian anticipates that incorporating quantum-mechanical principles into microscopy experiments might allow researchers to secure an even closer view.
The barriers of classical techniques could be bypassed by quantum science, he said.
More information: Anne M. Charrier et al, In situ plant materials hyperspectral imaging by multimodal scattering near-field optical microscopy, Communications Materials (2021). DOI: 10.1038/s43246-021-00166-7