The protection offered by COVID-19 vaccines has been diminished by the emergence of new strains of the coronaviruses. A new approach developed by researchers at the Indian Institute of Science renders viruses inactive.
In a study published in Nature Chemical Biology, the researchers report the design of a new class of artificial peptides or miniproteins that can not only block virus entry into our cells but also clump virions together.
A lock and a key are similar to aprotein-protein interactions. A lab-made miniprotein that mimics, competes with, and prevents the "key" from binding to the "lock" can affect this interaction.
The team used this approach to design miniproteins that can bind to and block the spikeProtein on the surface of the SARS-CoV-2 virus. The binding was further characterized by various methods.
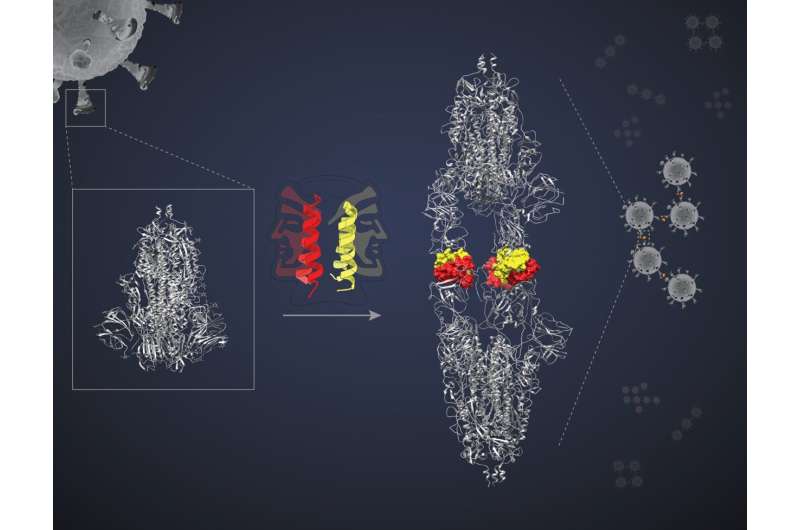
Each of these mini-proteins can pair up with another of its kind to form a dimer. Two faces are presented to interact with two targets. The researchers believed that the two faces would bind to two different targets and block their actions. Jayanta Chatterjee is the lead author of the study and is an associate professor in the MBU. The team decided to use a miniprotein called SIH-5 to target the interaction between the Spike and the ACE2 in human cells.
A trimer consists of three identical polypeptides. Each polypeptide has aRBD that binding to the ACE2receptor on the cell surface. This interaction leads to the entry of Viruses into the Cell.
SIH-5 was designed to block the binding of theRBD to humanACE2 One of the faces of the SIH-5 dimer was bound to one of the threeRBDs on the Sprotein trimer. The miniprotein was able to block both SProteins at the same time. There are several monomers that can block their targets. Their action is blocked a lot more effectively by cross-linking of S proteins. The avidity effect is what it is.
The SIH-5 appeared to be attached head-to-head. We were expecting a complex of one spike trimer. One of the authors says that he saw a structure that was more long. The spike proteins were being forced to form complexes with the miniprotein. Multiple spike proteins of the same virus can be inactivated by clumping. I have observed the antibodies against the spikeprotein under a cryo-EM. They didn't create any of the spikes.
It can be stored for months at room temperature without degrading.
The next step was to find out if SIH-5 could be used to prevent COVID-19 infections.
The miniprotein was found to be safe after being tested in the lab. In the lab of Raghavan Varadarajan, Professor at M BU, hamsters were dosed with the miniprotein and exposed to the coronaviruses. hamsters exposed only to the virus had much less cell damage in the lungs than these animals.
The researchers believe that with minor modifications and peptide engineering, this lab-made miniprotein could also be used to prevent otherProtein-Protein interactions.
More information: Bhavesh Khatri et al, A dimeric proteomimetic prevents SARS-CoV-2 infection by dimerizing the spike protein, Nature Chemical Biology (2022). DOI: 10.1038/s41589-022-01060-0 Journal information: Nature Chemical Biology