There are many chemical reactions that use a catalyst. It makes it possible for a molecule to be changed into another molecule without being changed itself. Precious metals make them expensive and potentially harmful to the environment. A catalyst made of iron has been designed to facilitate a chemical reaction called the Olefin metathesis reaction. Their work was published in nature catalysis.
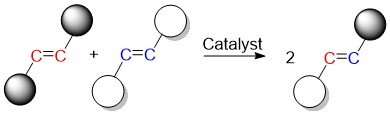
One of the most widely applicable catalysts for carbon-carbon double bond formation is the olefin metathesis reaction. Many chemical products contain carbon-carbon double bonds.
There are compounds with double bonds of carbon. New carbon-carbon double bonds are created when the carbon atoms in olefins are swapped. The catalyst breaks the double bonds and creates new ones.
ruthenium is a popular catalyst for this reaction. The goal of the study was to make the process cheaper and more eco-friendly by using iron as a catalyst. As ruthenium and iron are in the same group on the periodic table, they are expected to have similar properties.
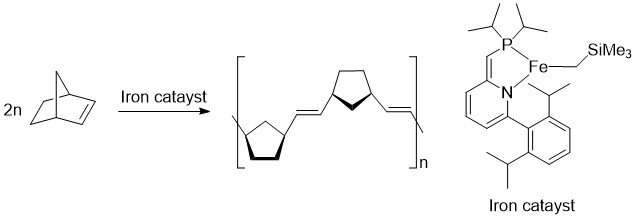
The researchers showed that a new iron complex could be used as a catalyst. They showed that it was possible to make a long chain molecule out of small chemical units.
The state-of-the-art ruthenium-based catalysts are still more applicable than the iron-based ones according to Takebayashi. The iron-catalyst isn't as active when it's exposed to air. The ruthenium one needs to be replaced before the iron-catalyst can do the job.
The study can be used by other researchers. I would like to see iron-based catalysts developed further.
More information: Satoshi Takebayashi, Iron-catalysed ring-opening metathesis polymerization of olefins and mechanistic studies, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00793-4. www.nature.com/articles/s41929-022-00793-4 Journal information: Nature Catalysis