Ingrid Fadelli is a writer for Phys.org.
A molecule will cool down when it is free in cold space. Physicists have shown that the cooling process can be accelerated and slowed down by the molecule.
Researchers at the Max-Planck Institute for Nuclear Physics in Germany and the Columbia Astrophysics Laboratory have recently carried out an experiment to measure the rate of quantum transitions. The first experimental evidence of this rate was offered by their findings, which were published in Physical Review Letters.
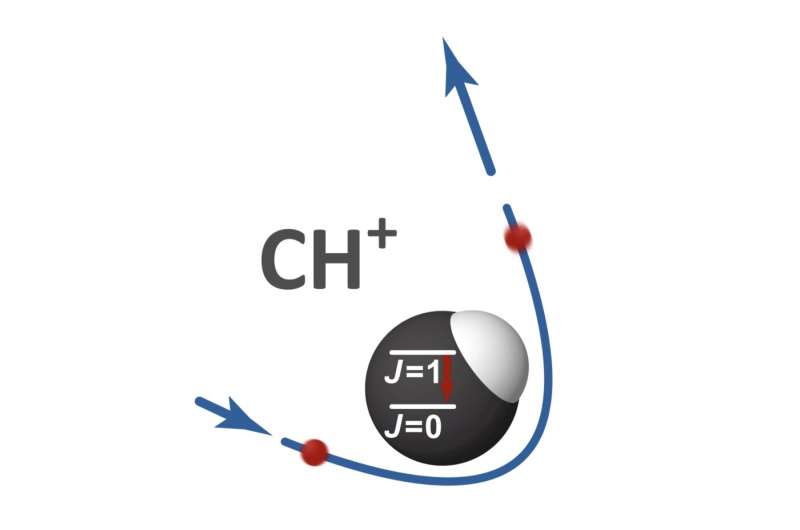
The lowest quantum level populations of the molecule can be changed in a collision process when electrons and ionized gases are present. The attractive force between the negatively charged electrons and the positively charged molecular ion makes the process of electronic collisions particularly efficient.
Physicists have been trying to figure out the strength of a free electron interacting with a molecule during a collision in order to change the state of the molecule. Their predictions have not been tested in an experimental setting.
No measurement could determine the effectiveness of the rotational level changes for a given electron density and temperature.
To collect this measurement, Klosi and his colleagues brought isolated charged molecule in close contact with electrons. They were able to experiment with the theoretical hypotheses and predictions outlined in previous works.
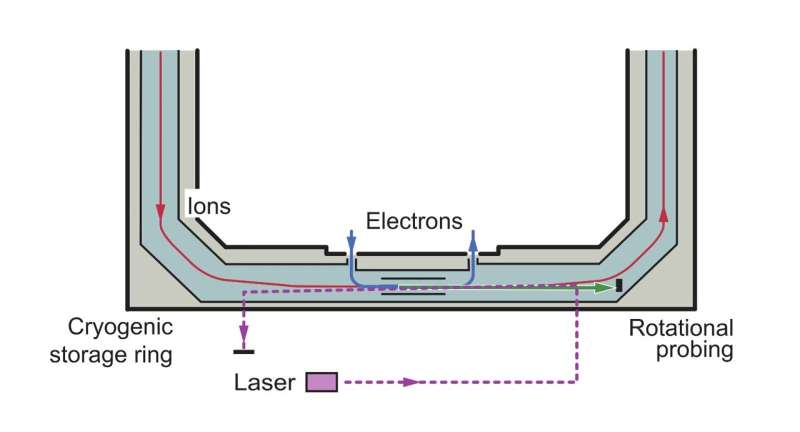
The Max-Planck Institute for Nuclear Physics in Germany was used as a storage ring for the experiment. In this ring, the molecule moves on a racetrack-like track in a high volume of gas.
The stored ion can be radiatively cool towards the temperature of the walls of the ring, generating ion that are populated in their lowest few quantum levels. The ion is stored in this ring for a long time, and a laser is used to analyze the ion's rotational energy.
The team could destroy a small fraction of the stored ion if they chose a specific wavelength for their probing laser. The fragments of the destroyed molecule were detected.
The team collected their data in both the presence and absence of electron collisions. They were able to detect population changes under the conditions set in their experiment.
To measure the process of rotating state changing collisions, one must ensure that the lowest rotational energy levels are in the molecular ion. The molecule can be isolated from the heat radiation of our environment.
In their experiment, Klosi and his colleagues were able to see that electron collisions dominated over radiative transitions. By using enough electrons, they could collect a quantitative measurement of electronic collisions.
The first experimental test of the existing theoretical predictions was provided by our measurements. We expect that future calculations will more strongly focus on the influence of electronic collisions on the lowest energy level populations in cold, isolated quantum systems.
The recent work by this team of researchers could have important research implications, as they confirmed theoretical predictions in an experimental setting for the first time. Their findings suggest that measuring the rates of quantum-level changes is important for analyzing faint signals of molecule in space detected by radio telescopes.
In the future, this paper could lead to new theoretical studies that consider the influence of electronic collisions on the occupation of rotational quantum levels in cold molecule. This could lead to more detailed experiments in this area if this helps to single out cases in which electronic collision have the strongest effects.
The plan is to introduce more versatile laser techniques to probe the rotational energy levels for more diatomic and polyatomic molecular species. This type of laboratory measurement will complement observational astronomy using the powerful observatories like the Atacama Large Millimeter/submillimeter Array in Chile.
More information: Ábel Kálosi et al, Laser Probing of the Rotational Cooling of Molecular Ions by Electron Collisions, Physical Review Letters (2022). DOI: 10.1103/PhysRevLett.128.183402 Journal information: Physical Review LettersThe Science X Network will be launched in 2022.