Last year, researchers at MIT's McGovern Institute for Brain Research discovered and characterized the first CRISPR enzyme that can make precise, guided cuts to strands of RNA without harming cells in the process. The team at the University of Tokyo has shown that it can be shrunk to a more compact version, making it an even more viable option for editing the RNA inside living cells. A detailed structural analysis of the original enzyme and a new, compact version of it were described in the journal Cell.
When we looked at the structure, it was clear there were some pieces that weren't needed, which we could actually remove, says Research Scientist and McGovern Fellow Omar Abudayyeh, who led the new work with Research Scientist and McGovern Fellow Jonathan Goo.
The authors, who include a former McGovern Institute postdoc Nathan Zhou and a University Tokyo graduate student, see the new three-dimensional structure of the enzymes as a rich resource to answer questions about the basic biology of the enzymes and reveal other ways to tinker with its function in the future.
Targeting the genes.
The ability to modify the genes inside human cells has given researchers the ability to research and develop new treatments for diseases. For some research and clinical purposes, editingRNA is more effective than altering DNA.
Any changes to a cell's genes are relatively permanent because a cell keeps its genes for life and passes an identical copy to daughter cells. The more short-lived molecule,RNA, is transcribed from DNA and degraded after a while.
There are lots of positives about being able to permanently change genes, especially when it comes to treating an inherited genetic disease.
When it recognized a particular gene, theCas13 was able to cut up all theRNA, but it had a messy side effect. This property makes it possible to detect the presence of a piece ofRNA in diagnostic tests, but not very useful for therapeutic use.
The discovery of a more precise form of RNA editing, similar to the Cas9 enzyme, opened the doors. The empty shell of a virus that researchers typically use to deliver gene editing machinery into patient's cells was too big to fit inside a single viral vector.
There is structural insight.
cryo- electron microscopy shines beams of electrons on frozen samples and measures how the beams are transmitted to determine the structure of the whole. The researchers were not sure how the parts of the single gene fit together.
The fascinating thing about the fusion of the different pieces of the same gene is that it should be all of them.
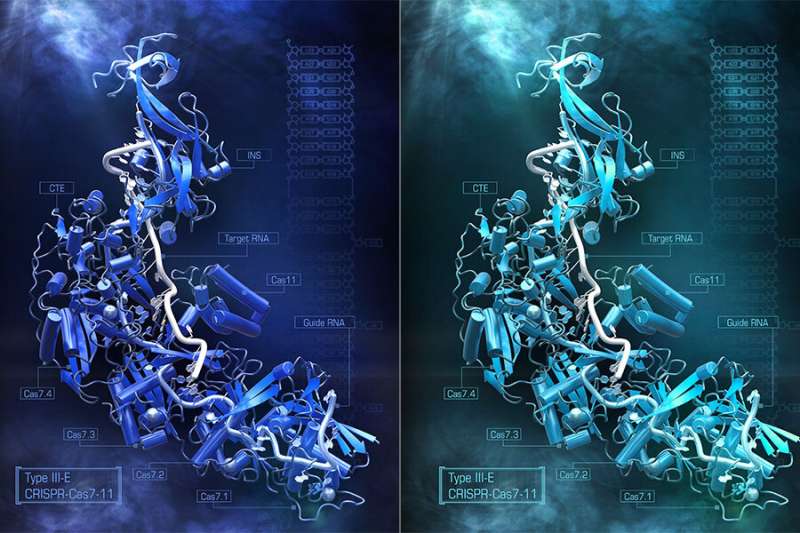
The structure of the ribonucleic acid binding molecule, caught in the act of binding both its target tRNA strand and the guide RNA, revealed how the pieces assembled and which parts of theProtein were critical to recognizing and cuttingRNA. This kind of structural insight is critical to figuring out how to make a certain number of jobs in human cells.
There was a section of theProtein that wasn't serving any apparent functional role illuminated by the structure. The researchers could remove it and re-engineering it to make it smaller without taking away its ability to targetRNA. A new compact version of the protein was created by removing different bits of this section. They packaged the system inside a single viral vector and delivered it into cells.
The team hopes to continue working towards the use of Cas7-11 for therapeutic applications, as well as future studies on other proteins that interact with it in the bacterium that it comes from.
Abudayyeh says that it is just the beginning of enabling that tool set.
More information: Kazuki Kato et al, Structure and engineering of the type III-E CRISPR-Cas7-11 effector complex, Cell (2022). DOI: 10.1016/j.cell.2022.05.003 Journal information: CellThe story was re-posted by MIT News, a popular site that covers news about MIT research, innovation and teaching.
Citation: Neuroscientists expand CRISPR toolkit with new, compact Cas7-11 enzyme (2022, May 31) retrieved 31 May 2022 from https://phys.org/news/2022-05-neuroscientists-crispr-toolkit-compact-cas7-.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.