Researchers have shown that aggregation of amyloid-beta causes cells tofry like eggs.
The researchers from the University of Cambridge used small and sensitive sensors to detect temperature changes inside individual cells, and found that amyloid-beta misfolds and clumps together, causing cells to overheat.
The researchers found that the heat released by amyloid-beta aggregation could cause other, healthy amyloid-beta to aggregate, causing more and more aggregates to form.
The researchers showed that amyloid-beta aggregation can be stopped and that the cell temperature can be lowered with the addition of a drug compound. The compound has the potential to be a therapeutic for Alzheimer's disease, but extensive tests and clinical trials are required before it can be used.
The researchers say their test could be used to diagnose Alzheimer's disease or to screen potential drug candidates. The results are reported in a journal.
Alzheimer's disease affects 44 million people worldwide and there are currently no effective treatments. Alzheimer's disease causes brain cells to die and the brain to shrink by building up tangles and plaques. This results in memory loss and personality changes.
It is difficult to study the disease since it develops over decades and a definitive diagnosis can only be given after examining brain tissue after death. It's not known what kind of changes inside a cell lead to amyloid-beta aggregation.
The research group at Cambridge's Department of Chemical Engineering and Biotechnology is looking into the link between temperature and amyloid-beta aggregation in human cells.
The field of studying temperature changes inside a cell is called intracellular thermogenesis. Scientists have developed sensors that can measure temperature changes, however, no one has ever tried to use them to study Alzheimer's disease.
The study's first author said that thermogenesis may promote further aggregation.
Kaminski Schierle, who led the research, said that heating a cell is like frying an egg.
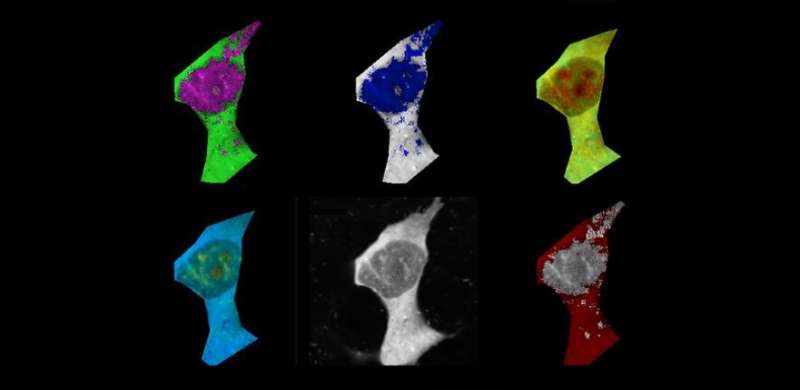
The link between aggregation and temperature was studied by the researchers using tiny temperature sensors. They added amyloid-beta to human cell lines to start the aggregation process and used a chemical called FCCP as a control.
The average temperature of the cells started to rise as amyloid-beta started to form fibrils. The increase in temperature was significant compared to cells that did not have amyloid-beta added.
Kaminski Schierle said that as the fibrils start elongating, they release energy in the form of heat.
The link between temperature and aggregation in live cells has not been shown before.
The researchers were able to identify the fibrils as the cause of thermogenesis using a drug. It had previously been unknown if the damage to thebatteries was caused by the aggregation of genes or by the power cells.
The researchers found that the rise in cellular temperatures could be mitigated by treating them with an aggregation inhibitor, a potential therapy for Alzheimer's disease.
Computational modeling was used to describe what might happen to amyloid-beta in an intracellular environment and why it might lead to an increase in temperatures. The researchers hope their work will inspire new studies.
More information: Chyi Wei Chung et al, Intracellular Aβ42 Aggregation Leads to Cellular Thermogenesis, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c03599 Journal information: Journal of the American Chemical Society Citation: Alzheimer's disease causes cells to overheat and 'fry like eggs' (2022, May 31) retrieved 31 May 2022 from https://phys.org/news/2022-05-alzheimer-disease-cells-overheat-eggs.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.