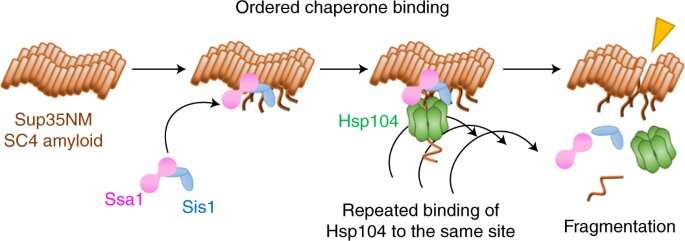
In a finding that has important ramifications for neurodegenerative disorders such as Alzheimer's disease, RIKEN researchers have discovered how three chaperone molecule in yeast cells work together to break up amyloid fibrils.
The wrong way to fold the proteins can affect their performance. prions are particularly harmful because they pass on their distorted shapes to the proteins around them, which then mesh together to form robust rafts called amyloid fibrils. Amyloid fibrils have been implicated in prion diseases, which are always fatal, and neurodegenerative disorders such as Alzheimer's and Parkinson's diseases.
The process by which amyloid fibrils form has received a lot of attention, but less is known about the mechanism by which they are broken up in cells. The process of disaggregation could allow prions to spread between cells.
Many researchers have investigated the formation mechanism of amyloid fibrils, but there have been very few studies of amyloid disaggregation.
Knowing how amyloid fibrils are broken up could help researchers develop treatments for prion and neurodegenerative diseases.
A group of people, led by Yoshiko Nakagawa, have used a form of single-molecule fluorescence microscopy to film how three molecules coordinate to break up amyloid fibrils in a prion system in yeast.
The break-up begins with two chaperones, Hsp70 and Hsp40, binding to the fibril. A third chaperone, Hsp 104, repeatedly binding to the same site, chips away at the fibril.
The team found that a dissolution mechanism also breaks up fibrils by a different chaperone. The shape of the fibril is what determines which mechanism occurs.
He says that there were many surprises in the study.
In the future, the team hopes to achieve atomic resolution by exploring the mechanisms in greater detail.
More information: Yoshiko Nakagawa et al, Amyloid conformation-dependent disaggregation in a reconstituted yeast prion system, Nature Chemical Biology (2022). DOI: 10.1038/s41589-021-00951-y Journal information: Nature Chemical Biology Citation: Three chaperones coordinate the breakup of amyloid fibrils in yeast (2022, May 26) retrieved 27 May 2022 from https://phys.org/news/2022-05-chaperones-breakup-amyloid-fibrils-yeast.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.