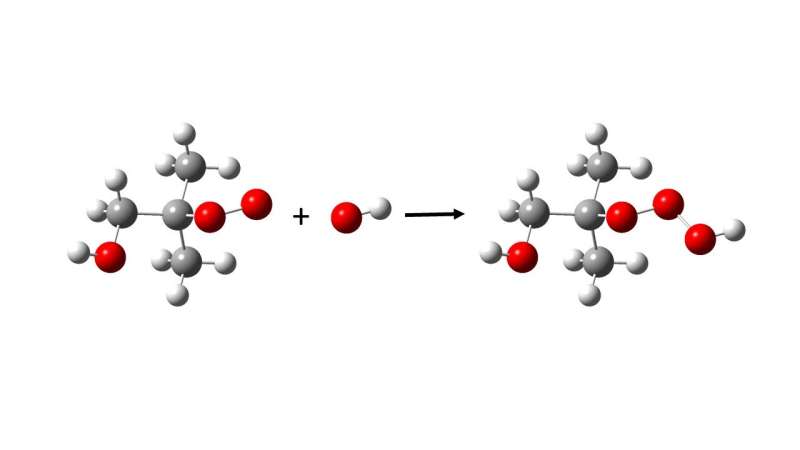
For the first time, a new class of super-reactive chemical compounds has been discovered. The formation of so-called trioxides, an extremely oxidizing chemical compound that likely affects both human health and our global climate, has been documented by researchers from the University of Copenhagen in close collaboration with international colleagues.
Hydrogen peroxide is a chemical compound. All peroxides have two oxygen atoms attached to each other, making them highly reactive and often dangerous. They are used for a lot of things, from lightening teeth to cleaning wounds. peroxides are found in the atmosphere.
There has been speculation as to whether trioxides, chemical compounds with three oxygen atoms attached to each other, and therefore even more reactive than the peroxides, are found in the atmosphere as well. It has never been proven.
This is what we have accomplished, says Professor Henrik Grum Kjærgaard, at the University of Copenhagen's Department of Chemistry. The type of compounds we discovered are unique to them. Because they are extremely oxidizing, they most likely bring a host of effects that we have yet to uncover.
There is a completely new class of chemical compounds called hydrotrioxides. The researchers at the University of Copenhagen, together with colleagues at the Leibniz Institute for Tropospheric Research (TROPOS) and the California Institute of Technology, have demonstrated that these compounds are formed under atmospheric conditions.
The researchers have shown that isoprene and dimethyl sulfide are formed during the decomposition of other substances.
It is quite significant that we can now show that these compounds form in the atmosphere and that they are formed from almost all chemical compounds. The second author of the study says that all speculation must be put to rest.
There are two types of radicals. The lifespans of most chemical compounds are expected to range from minutes to hours, according to the researchers. They are stable enough to react with many other atmospheric compounds.
It is possible that absorbed into aerosols.
The research team believes that the trioxides can penetrate into tiny airborne particles, known as aerosols, which pose a health hazard and can lead to respiratory and cardiovascular diseases.
They will most likely enter aerosols, where they will form new compounds with new effects. It's easy to imagine that aerosols can be harmful if breathed in. Further investigation is needed to address potential health effects.
Aerosols are one of the things that are hard to describe in climate models. According to the researchers, there is a high chance that hydrotrioxides impact how many aerosols are produced.
The ratio of sunlight that Earth absorbs and sends back into space is affected by the amount of sunlight reflected and absorbed by aerosols. When aerosols absorb substances, they grow and contribute to cloud formation, which affects Earth's climate as well.
The effect of compound needs to be studied further.
The researchers hope that the discovery of hydrotrioxides will help them understand the effects of the chemicals we emit.
Most human activity causes emission of chemical substances into the atmosphere. Knowledge of the reactions that determine atmospheric chemistry is important if we are to be able to predict how our actions will affect the atmosphere in the future.
The compounds have been around for a long time and neither he nor the other man are worried about them. The fact that the compounds are formed and live for a certain amount of time means that it is possible to study their effect in a more targeted way and respond if they turn out to be dangerous.
The discovery suggests that there could be more than we know about. The air surrounding us is full of complex chemical reactions. If we want to get better at finding solutions, we need to keep an open mind.
More information: Torsten Berndt et al, Hydrotrioxide (ROOOH) formation in the atmosphere, Science (2022). DOI: 10.1126/science.abn6012. www.science.org/doi/10.1126/science.abn6012 Journal information: Science Citation: New type of extremely reactive substance discovered in the atmosphere (2022, May 26) retrieved 26 May 2022 from https://phys.org/news/2022-05-extremely-reactive-substance-atmosphere.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.