Scientists around the world are increasingly committed to addressing the issue of greenhouse gases. Many research groups focus on carbon dioxide or methane revalorization, but a team led by Dr. Josep Cornell has a different approach.
The global warming potential of nitrous oxide is 300 times higher than that of carbon dioxide. In recent decades, emissions of nitrous oxide have increased due to human activities.
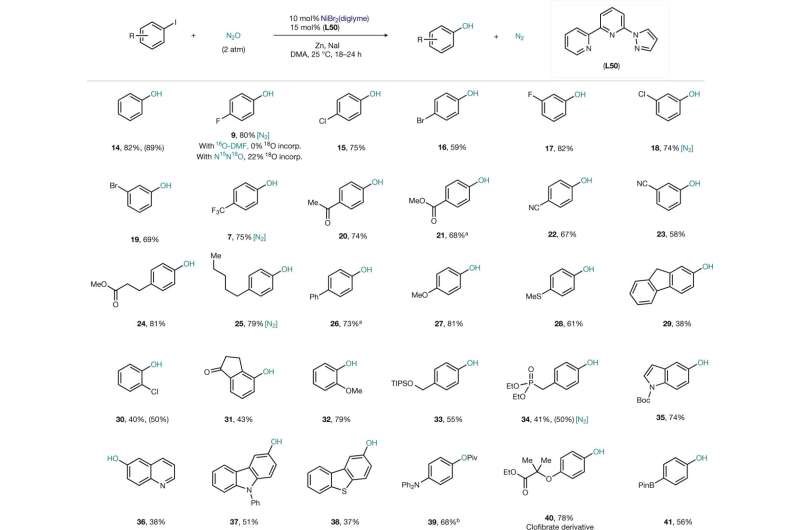
Josep Cornell's group considers this molecule too valuable to be released into the atmosphere. N 2 O is a great source of O atoms, and the byproduct generated is harmless. The challenge was that for a long time, N 2 O was considered a gas that needed to be grabbed from its structure. The team at the Cornell lab has shown that reacting N 2 O with a simple catalyst can be used to make phenols.
The paper "Catalytic synthesis of phenols with nitrous oxide" was published in Nature.
More information: Franck Le Vaillant et al, Catalytic synthesis of phenols with nitrous oxide, Nature (2022). DOI: 10.1038/s41586-022-04516-4 Journal information: Nature Citation: Catalytic synthesis of phenols with nitrous oxide (2022, May 9) retrieved 9 May 2022 from https://phys.org/news/2022-05-catalytic-synthesis-phenols-nitrous-oxide.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.