Dylan Thomas said that the force that through the green fuse drives the flower.
Plants are able to eat sunlight. One of the grand challenges of photochemical research is the use of light to make new molecule in the lab instead of the leaf.
It's new again. Sometimes a return to old tools is required in order to gain new insight. A century-old microwave technique has been revived by a team from the National Renewable Energy Laboratory.
"Ion-pair reorganization regulates reactivity in photoredox catalysts" was published in Nature Chemistry. The work was done as part of the renewable and sustainable energy institute.
This work, which is part of a U.S. Department of Energy (DOE) Energy Frontier Research Center, reveals how an important class of "photoredox catalysts" operate.
Photoredox catalysis is a branch of photochemistry that uses light to drive chemical reactions. It's possible to drive reactions with high barriers and better control over the final product.
A detailed understanding of how the reaction works is required to use these new reactions.
The work was started to learn how charges move. The first catalyst we studied was unexpected.
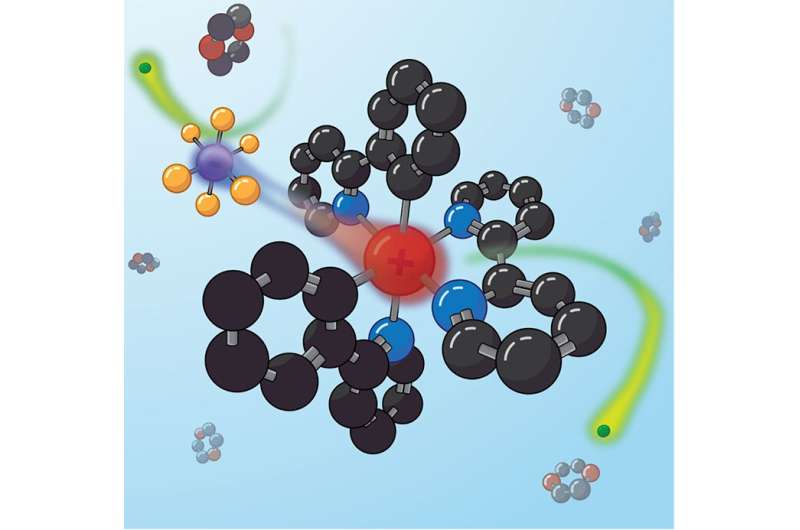
The NREL team studied a catalyst made of two halves. The charge was added to zero because one half was positive and the other negative. The positive side was thought to do all the work in light-driven chemical reactions, while the negative side was supposed to be a "counterion"
When the catalyst is excited by light, the counterion can block some reactions. Sometimes you need to get in the way. The counterion does that.
The team was able to show that the negatively charged counterion moved after the molecule was excited with light by measuring exactly how their microwave signal changed as it passed through the solution. The counterion can block a chemical reaction because the molecule needs a path for electrons to move through. The blocking action of the counterion resulted in four different reactions.
Although the measurement tool used by the NREL team has been around for over 100 years, scientists historically had to conduct time consuming control experiments to interpret their results That was changed by computers. Quantitative simulations of how the catalyst molecule rotates in solution were used by the NREL team.
More information: J. D. Earley et al, Ion-pair reorganization regulates reactivity in photoredox catalysts, Nature Chemistry (2022). DOI: 10.1038/s41557-022-00911-6 Journal information: Nature Chemistry