Most human hearts have the same muscles, blood vessels and orientations. Organs such as hearts or stomachs look the same and function the same across individual organisms because cells follow rigorous processes during development that get them exactly where they need to go.
The process of development involves a lot of steps. The lab of Caltech's Stathopoulos focuses on studying these steps. A model system for the lab is fruit flies, which have a 24 hour developmental cycle with observable changes almost every minute.
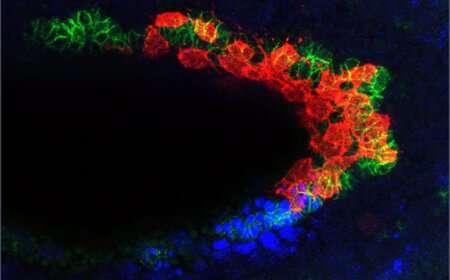
A new paper from the Stathopoulos laboratory looks at the cells that will eventually become muscle in fruit flies. The longest distance from the back of the embryo to the front is six hours.
There are mechanisms that ensure that any cells that wander will self-destruct. Many types of cancer can be a result of resistance to anokis. Understanding the pathways that guide healthy anoikis may give insight into how cancers spread and why certain parts of the body are invaded.
Stathopoulos believes that cell death is a normal part of development. The migrating cell has to decide if it's in the right place in the body. It needs to self-destruct if it isn't in the right spot. The pathways that the cell can use to do this have been determined.
The paper is online in the journal. Frank Macabenta is the study's first author.
The fruit fly embryo is not the only one that the CVM cells travel through. The 40 to 50 cells follow a track made up of different cell types. TVM cells emit a signal that lets a CVM cell know that it is in the right place.
The CVM cells have to navigate around a bend in the embryo at the end of their migration. When the time is right to start building muscle, CVM cells start growing at this juncture. Some cells drift off of the TVM track when they start to multiply. This is the place where the lost cells will self-destruct.
Anoikis is caused by a gene called hid. The cell dies when hid is expressed. The CVM cells do not die unless they fall off of the TVM track, according to the new work.
If a cell falls off of the track and ceases to receive FGF signals, it will die, but it can stay alive if it stays on track. It is possible for the embryo to make sure that the cells are functioning and not self-destructing.
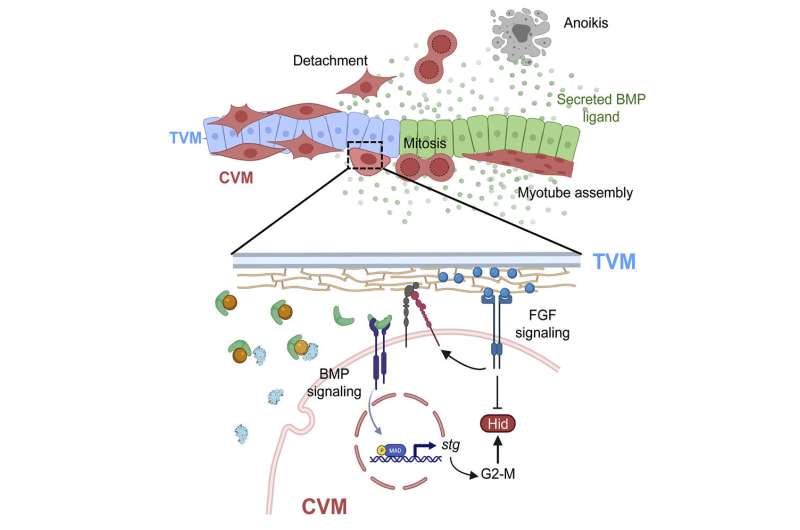
The team discovered that the BMP pathway controls when the cells start to grow. Cells navigate the U-shaped turn at the halfway point of their migration. This signals allows cells to divide and grow.
Cells have an internal clock, known as the cell cycle, which controls the timing of growth and other processes. When the cell cycle is disrupted, hid is no longer expressed at the right point. Cells that fail to divide are not able to express hid in a timely manner, so timing the exact expression of hid is important.
It is important that cells have these quality control mechanisms because stray cells can damage the rest of the organisms.
The cells that came off the track would survive and wreak havoc on the central nervous system, where they really shouldn't be. They go back to a kind of "plan B" where they find a location they like. When you look at autopsies of people who have had cancer, the metastasizing cells will colonize certain areas. Understanding how this works, how cells go awry and figuring out the'second-best' signals to follow is part of our research. We want to know what other signals the CVM cells are following that lead them to the central nervous system. It's possible that certain types of metastases preferentially colonize other tissues.
More information: Frank Macabenta et al, BMP-gated cell-cycle progression drives anoikis during mesenchymal collective migration, Developmental Cell (2022). DOI: 10.1016/j.devcel.2022.05.017